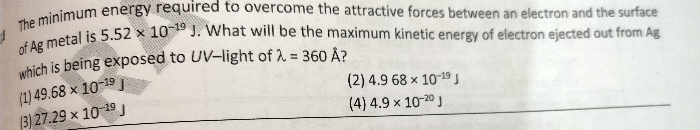CBSE Class 11-science Answered
Light of wavelength 1281.8nm is emitted when an electron of hydrogen atom drops from 5th to 3rd energy level calculate the wavelength of the photon emitted when electron falls from 3rd to ground level.
Asked by Taniyarana150 | 23 May, 2019, 00:01: AM
Energy change in emission is calculated by-
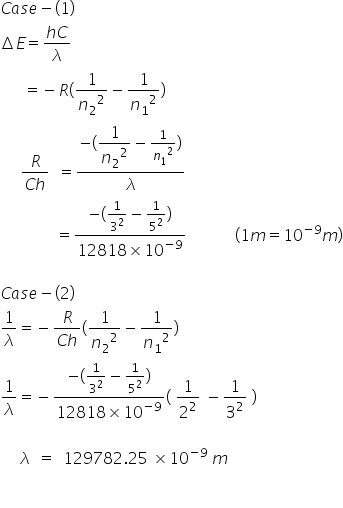
Answered by Ravi | 23 May, 2019, 12:50: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by sheeltahiliani | 23 May, 2024, 11:58: AM
CBSE 11-science - Chemistry
Asked by saranyachakraborty2007 | 25 Apr, 2024, 05:23: AM
CBSE 11-science - Chemistry
Asked by habibakhatoon112 | 15 Jul, 2022, 21:14: PM
CBSE 11-science - Chemistry
Asked by akankhyapradhan123 | 22 Oct, 2021, 19:29: PM
CBSE 11-science - Chemistry
Asked by kusumghosh71 | 21 Aug, 2021, 10:59: AM
CBSE 11-science - Chemistry
Asked by muramshettyaashrith218 | 16 Sep, 2020, 21:58: PM
CBSE 11-science - Chemistry
Asked by nandanappillai | 21 May, 2020, 13:08: PM
CBSE 11-science - Chemistry
Asked by rajarage18 | 23 Jan, 2020, 20:00: PM
CBSE 11-science - Chemistry
Asked by kuldeepsingh33087 | 27 Jul, 2019, 11:35: AM
CBSE 11-science - Chemistry
Asked by vijaykumartecno348 | 06 Jul, 2019, 21:26: PM