CBSE Class 12-science Answered
derive the nernst eqn for daniel cell
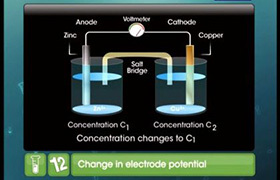
For Daniel cell,
Zn(s)+Cu2+(aq) <=> Zn2+(aq) + Cu(s)
Zn(s)|Zn2+(aq)||Cu2+(aq)|Cu
In Daniell cell, the electrode potential for any given concentration of Cu2+ and Zn2+ ions, we write
For Cathode:
E(Cu2+/Cu) = E0(Cu2+/Cu) – RT/2F ln(1/[Cu2+(aq)]
For Anode:
E(Zn2+/Zn) = E0(Zn2+/Zn) – RT/2F ln(1/[Zn2+(aq)]
The cell potential, Ecell = E(Cu2+/Cu) - E(Zn2+/Zn)
= E0(Cu2+/Cu) – RT/2F ln(1/[Cu2+(aq)] - E0(Zn2+/Zn) + RT/2F ln(1/[Zn2+(aq)]
= E0(Cu2+/Cu) -E0(Zn2+/Zn)- RT/2F ln(1/[Cu2+(aq)] + RT/2F ln(1/[Zn2+(aq)]
= E0(Cu2+/Cu) -E0(Zn2+/Zn)- RT/2F (ln(1/[Cu2+(aq)]- ln(1/[Zn2+(aq)])
Therefore, Nernst equation for Daniel cell is
Ecell = E0cell - RT/2F ln [Zn2+]/[Cu2+]
Ecell = E?cell − 2.303RT/2F ln [Zn2+]/[Cu2+]