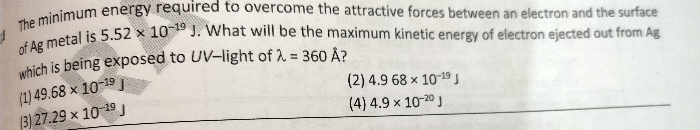CBSE Class 11-science Answered
Calculate the frequency and the wavelength of the radiation in nannometers emmitted when an electron in the hydrogen atom jumps from 3rd orbit to the ground state. In which region of the electromagnetic spectrum will this line lie.
Asked by Keshav sultania | 14 Sep, 2013, 12:48: PM
Frequency = R ( 1/ n1^2 - 1/ n2 ^2)
Here R is Rydberg Constant = 1.09766 * 10^ 7 m ^-1
n1 = 1 and n2 = 3. We put the values in formula and we get the frequency = 9.75 *10 ^ 6 m^-1
and wavelength = 1/ frequency = 1.02 * 10 ^ -7 meter. The Spectrum will lie in Layman series.
Answered by | 14 Sep, 2013, 09:33: PM
Application Videos
Concept Videos
CBSE 11-science - Chemistry
Asked by saranyachakraborty2007 | 25 Apr, 2024, 05:23: AM
CBSE 11-science - Chemistry
Asked by ammu32811 | 20 Feb, 2024, 08:58: AM
CBSE 11-science - Chemistry
Asked by ee7511641 | 13 Jan, 2024, 03:37: PM
CBSE 11-science - Chemistry
Asked by kv3582976 | 11 Oct, 2023, 06:57: AM
CBSE 11-science - Chemistry
Asked by o230397 | 23 Sep, 2023, 02:48: PM
CBSE 11-science - Chemistry
Asked by shrreya27harshitha | 17 Jul, 2022, 04:15: PM
CBSE 11-science - Chemistry
Asked by habibakhatoon112 | 15 Jul, 2022, 09:14: PM
CBSE 11-science - Chemistry
Asked by deba.biswas561 | 14 Jun, 2022, 08:07: AM
CBSE 11-science - Chemistry
Asked by advssdrall | 12 Jan, 2022, 05:12: AM
CBSE 11-science - Chemistry
Asked by ks1221516 | 14 Nov, 2021, 06:46: PM