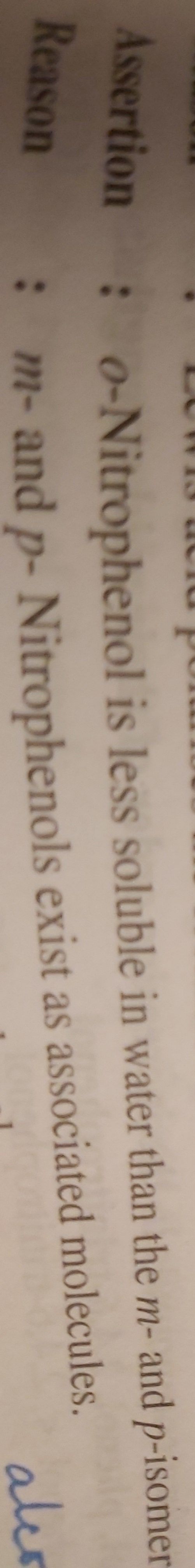CBSE Class 12-science Answered
An Organic compound (A) on treatment with acetic acid in the presence of sulphuric acid produces an ester (B) . (A) on mild oxidation gives (C) . (C) With 50% KOH followed by adification by dil. HCL generates (A) & (D) . (D) With PCl5 followed by reaction with ammonia gives (E) . (E) on dehydration produces hydrocyanic acid . identify compounds A,B,C,D,E
Also explain ,why do phenols not give the protonation reaction readily ?
Asked by yash.bisht9910 | 16 Jun, 2015, 01:08: PM
Dear yash.bisht9910@gmail.com
Thanks for asking us a question in Ask the Expert section of TopperLearning.com.
We cannot entertain more than 2 questions in a single query. In case of multiple questions within a query, please post each question individually and let us know where you are getting stuck so that we would be able to explain things better.
Answer to your first query is posted below:
Regards
Topperlearning Team.
Answered by Vaibhav Chavan | 16 Jun, 2015, 10:05: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by kavitabawane190 | 08 Mar, 2024, 05:24: PM
CBSE 12-science - Chemistry
Asked by rp0055293 | 07 Feb, 2024, 08:28: AM
CBSE 12-science - Chemistry
Asked by vipulverma | 14 Feb, 2022, 04:44: PM
CBSE 12-science - Chemistry
Asked by kaziryan.05 | 23 Jun, 2021, 08:02: PM
CBSE 12-science - Chemistry
Asked by Rg598555 | 30 Oct, 2019, 10:35: PM
CBSE 12-science - Chemistry
Asked by jain.pradeep | 30 Aug, 2019, 04:13: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 27 Mar, 2014, 03:43: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 07 Jun, 2016, 03:12: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 07 Jun, 2016, 03:17: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 27 Mar, 2014, 04:54: PM