CBSE Class 12-science Answered
Among noble gases and group 16 elements which one has higher ionisation enthalpy and why?
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
Among noble gases and group 16 elements, the noble gases have a higher ionisation enthalpy because they have stable electronic configurations i.e., they have eight electrons in their valence shell.
Answered by | 04 Jun, 2014, 03:23: PM
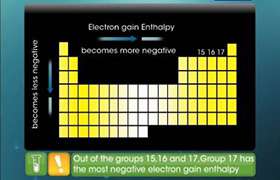
Concept Videos
CBSE 12-science - Chemistry
Asked by skumkum976 | 08 May, 2021, 03:49: PM
CBSE 12-science - Chemistry
Asked by manivannanbalakrishnan52 | 09 Dec, 2020, 10:06: PM
CBSE 12-science - Chemistry
Asked by onkaronkar618 | 12 Oct, 2020, 11:38: PM
CBSE 12-science - Chemistry
Asked by contactus.topperlearning | 13 Sep, 2020, 01:21: PM
CBSE 12-science - Chemistry
Asked by Daisysnaitz | 24 Apr, 2020, 01:07: AM
CBSE 12-science - Chemistry
Asked by minipkda | 22 May, 2018, 06:42: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 09 Jun, 2014, 04:03: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 07 Jun, 2016, 11:05: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 09 Jun, 2014, 04:38: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 10 Jun, 2014, 09:24: AM