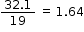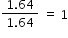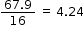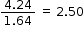CBSE Class 11-science Answered
a floride of oxygen was prepared by mixing oxygen and flourine in the proper ratio.This compound cantains 32.1%flourine and 67.9%oxygen. what is the empirical formula of the compound? (at. wt of f =19 and o=16)
Asked by nileshpatel19752000 | 19 Sep, 2016, 06:05: PM
Solution:
|
Element |
Percentage by mass |
Relative Number of Moles |
Simple Ratio of Moles |
Simplest Whole Number Ratio |
|
F |
32.1 |
|
|
1 × 2 = 2 |
|
O |
67.9 |
|
|
2.5 × 2 = 5 |
Simplest Whole number ratio is 2 : 5
Hence the empirical formula of compound is: O5F2
Answered by Hanisha Vyas | 19 Sep, 2016, 07:03: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by drhimasingh | 22 May, 2020, 11:39: AM
CBSE 11-science - Chemistry
Asked by nareshrajpurohit43109 | 22 May, 2020, 11:18: AM
CBSE 11-science - Chemistry
Asked by d6knx7qmw1 | 15 May, 2020, 10:37: PM
CBSE 11-science - Chemistry
Asked by sahadipa1975 | 02 May, 2020, 08:53: AM
CBSE 11-science - Chemistry
Asked by abhishek19362771 | 08 Apr, 2020, 03:48: PM
CBSE 11-science - Chemistry
Asked by anilsolanki2060 | 22 Feb, 2020, 10:12: AM
CBSE 11-science - Chemistry
Asked by pujakurmi22 | 11 Nov, 2019, 10:59: PM
CBSE 11-science - Chemistry
Asked by jkatwara | 14 Oct, 2019, 12:21: PM
CBSE 11-science - Chemistry
Asked by vikas.kochhar6 | 30 Aug, 2019, 03:58: PM
CBSE 11-science - Chemistry
Asked by pb_ckt | 19 May, 2019, 11:56: PM