CBSE Class 11-science Answered
2g Feso4 is completely oxidised by 0.05M kmno4.what volume of kmno4 is required?
Asked by nareshrajpurohit43109 | 22 May, 2020, 11:18: AM
The reaction is,
10FeSO4 + 2KMnO4 + 8H2SO4 → K2 SO4 + 2MnSO4 + 5Fe2(SO4)3 + 8H2O
(10×151.8) (2×158)
10×151.8 gm of FeSO4 require KMnO4 = 2×158 g
2 gm of FeSO4 will require KMnO4 = 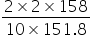
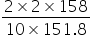
Let V ml of 0.05 M KMnO4 solution is required,
Amount of KMnO4 in the solution 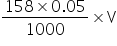
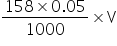
Thus, 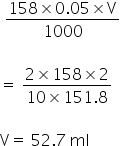
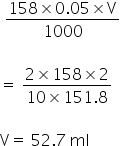
Answered by Varsha | 22 May, 2020, 16:40: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by rathodhamirbhai94 | 12 Jul, 2024, 21:34: PM
CBSE 11-science - Chemistry
Asked by drhimasingh | 22 May, 2020, 11:39: AM
CBSE 11-science - Chemistry
Asked by nareshrajpurohit43109 | 22 May, 2020, 11:18: AM
CBSE 11-science - Chemistry
Asked by d6knx7qmw1 | 15 May, 2020, 22:37: PM
CBSE 11-science - Chemistry
Asked by sahadipa1975 | 02 May, 2020, 08:53: AM
CBSE 11-science - Chemistry
Asked by abhishek19362771 | 08 Apr, 2020, 15:48: PM
CBSE 11-science - Chemistry
Asked by anilsolanki2060 | 22 Feb, 2020, 10:12: AM
CBSE 11-science - Chemistry
Asked by pujakurmi22 | 11 Nov, 2019, 22:59: PM
CBSE 11-science - Chemistry
Asked by jkatwara | 14 Oct, 2019, 12:21: PM
CBSE 11-science - Chemistry
Asked by vikas.kochhar6 | 30 Aug, 2019, 15:58: PM