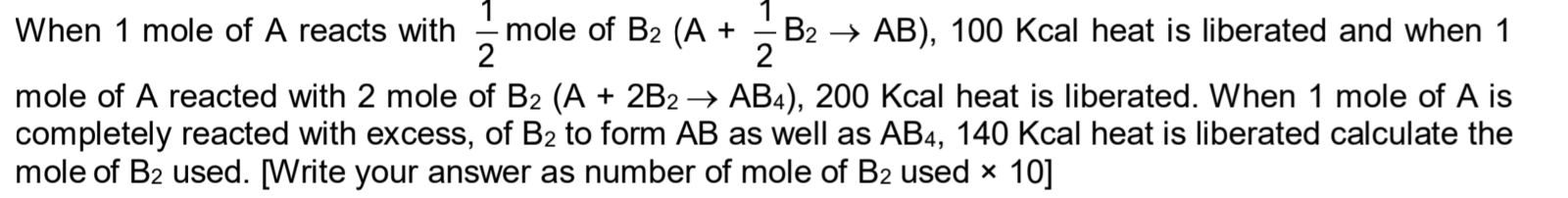CBSE Class 11-science Answered
100 g CaCO3 reacts with 1L,1M HCl. How much CO2 will be obtained on complete reaction
Asked by Anil | 16 May, 2017, 10:07: PM
Chemical reaction: CaCO3 + 2HCl → CaCl2 + CO2 + H2O
Moles in 100 g CaCO3 = 100 g / 100 g mol-1 = 1 mol
Since molarity of a solution means the number of moles of solute in 1 litre of solution. The number of moles in 1 L, 1 M HCl = 1 mol
In the above reaction, for 1 mol of CaCO3 we require 2 mol of HCl, but we have only 1 mol of HCl is a limiting reactant.
As 2 moles of HCl gives 1 mol of CO2, 1 mol of HCl will produce = (1/2) x 1 = 0.5 mol of CO2.
Hence, on complete reaction, 0.5 mol or 22 g (0.5 mol x 44 g mol-1) of CO2 will be obtained.
Answered by Prachi Sawant | 17 May, 2017, 01:49: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by drhimasingh | 22 May, 2020, 11:39: AM
CBSE 11-science - Chemistry
Asked by nareshrajpurohit43109 | 22 May, 2020, 11:18: AM
CBSE 11-science - Chemistry
Asked by d6knx7qmw1 | 15 May, 2020, 10:37: PM
CBSE 11-science - Chemistry
Asked by sahadipa1975 | 02 May, 2020, 08:53: AM
CBSE 11-science - Chemistry
Asked by abhishek19362771 | 08 Apr, 2020, 03:48: PM
CBSE 11-science - Chemistry
Asked by anilsolanki2060 | 22 Feb, 2020, 10:12: AM
CBSE 11-science - Chemistry
Asked by pujakurmi22 | 11 Nov, 2019, 10:59: PM
CBSE 11-science - Chemistry
Asked by jkatwara | 14 Oct, 2019, 12:21: PM
CBSE 11-science - Chemistry
Asked by vikas.kochhar6 | 30 Aug, 2019, 03:58: PM
CBSE 11-science - Chemistry
Asked by pb_ckt | 19 May, 2019, 11:56: PM