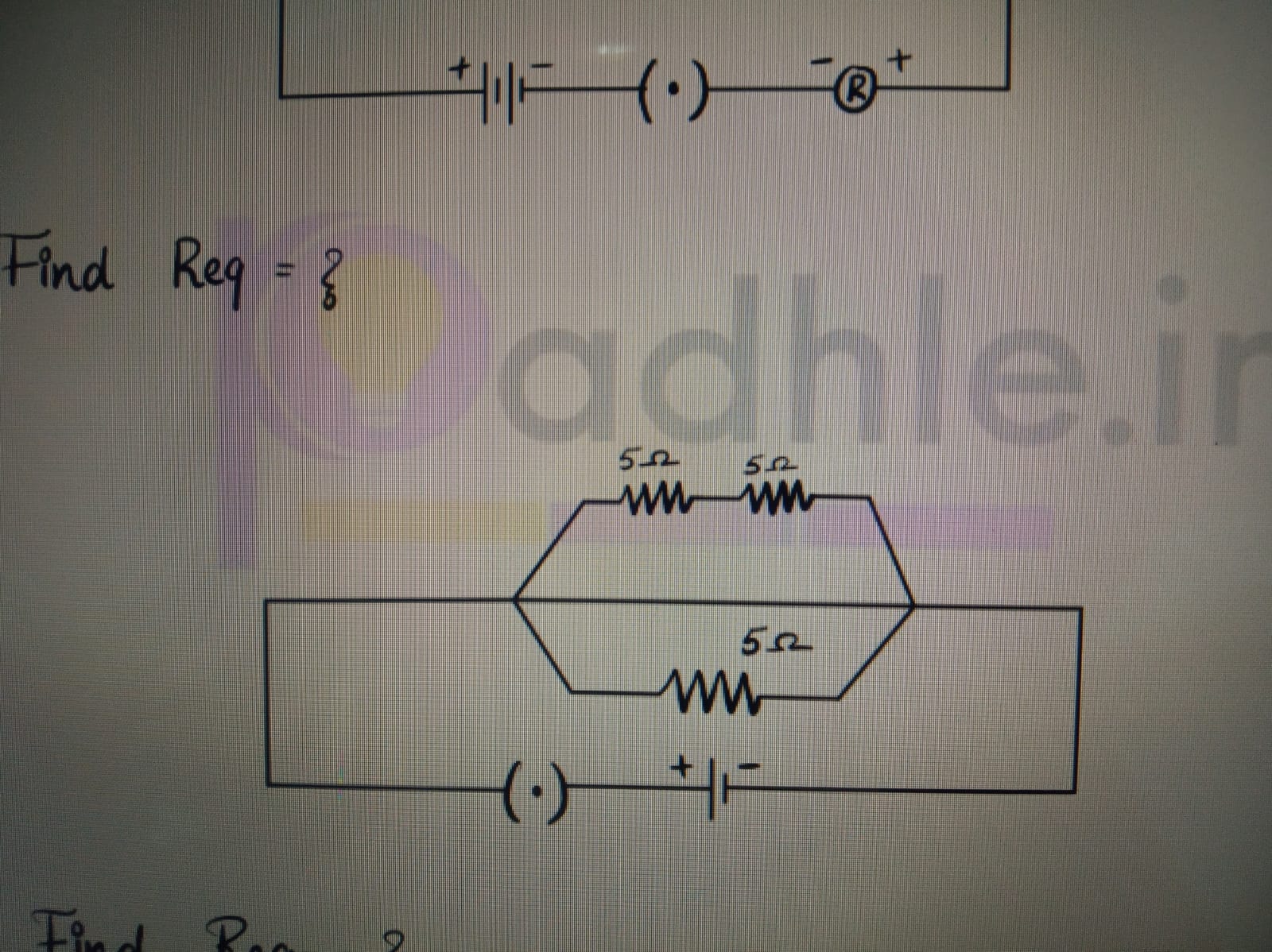
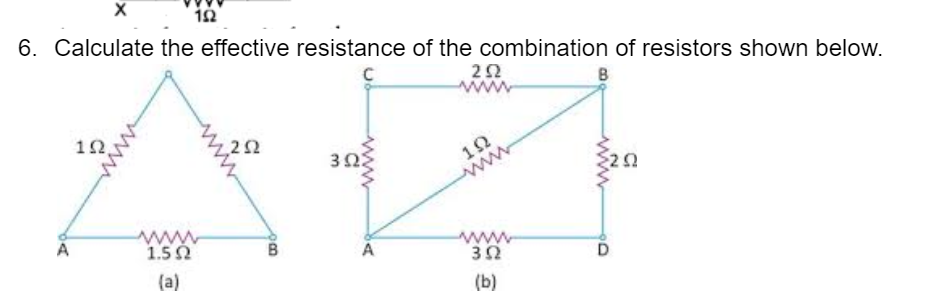
CBSE Class 10 Answered
1 C of charge is contained in = 6.25 x 1018 electrons . explain how.
Asked by Rhea Babu | 12 Jul, 2013, 11:51: PM
we shall use the following relation for quantisation of electric charge
q = ne
where
q is the total charge, here it is 1 Coulomb
n is the number of electric charges (or electrons) carrying that total charge
and e is the electronic charge or 1.6 x 10-19 C
now, the number of charge carrying electrons will be
n = q / e
or
n = 1 / 1.6 x 10-19
thus, by solving further, we get
n = 6.25 x 1018 number of electrons which constitute 1 Coulomb of charge
Answered by | 13 Jul, 2013, 11:01: AM
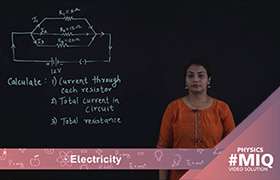
Application Videos
Concept Videos
CBSE 10 - Physics
Asked by khajannirwan | 27 Feb, 2024, 10:20: PM
CBSE 10 - Physics
Asked by saanviyadla | 24 Jan, 2024, 07:06: PM
CBSE 10 - Physics
Asked by kamalaranjanmohantymohanty5 | 06 Jan, 2024, 10:05: AM
CBSE 10 - Physics
Asked by nandhikasugumar | 05 Oct, 2023, 04:01: PM
CBSE 10 - Physics
Asked by daniya062008 | 02 Oct, 2023, 08:25: PM
CBSE 10 - Physics
Asked by prassanna.j | 03 Sep, 2023, 12:28: PM
CBSE 10 - Physics
Asked by prassanna.j | 03 Sep, 2023, 12:21: PM
CBSE 10 - Physics
Asked by prassanna.j | 03 Sep, 2023, 12:13: PM
CBSE 10 - Physics
Asked by prassanna.j | 03 Sep, 2023, 12:11: PM