ICSE Class 10 - Mole Concept Videos
Mole Concept and Stoichiometry
Mole Concept and Stoichiometry, Mole Concept
-
What will be the mass. ??

- How to calculate Emphirical formula and Molecular formula? thanks.
- In Chemistry, in a given reaction , how to recognize which reaction it is i.e oxidation,reduction or redox reaction?
-
b) one ..
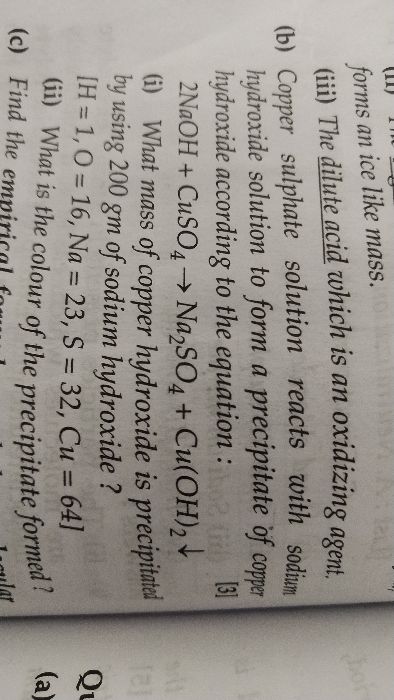
-
L

- Find the volume of oxygen at STP required for the complete combustion of 2 litres of carbon monoxide at S.T.P.
- why mole concept important
- A mixture of hydrogen and chlorine occupying 36 cm3 was exploded. On shaking it with water, 4cm3 of hydrogen was left behind. Find the composition of the mixture.
-
What volume of oxygen at STP is required to affect the combustion of 11 litres of ethylene [C2H4] at 273o C and 380 mm of Hg pressure?
C2H4+3O2
 2CO2 + 2H2O
2CO2 + 2H2O
- Calculate the number of molecules in 1 kg of Calcium Chloride.

