ICSE Class 10 Answered
In Chemistry, in a given reaction , how to recognize which reaction it is i.e oxidation,reduction or redox reaction?
Asked by rashikulkarni28 | 25 Jun, 2022, 22:24: PM
A chemical reaction in which loss of electrons and gain of electrons occur simultaneously is called a redox reaction.
Example:
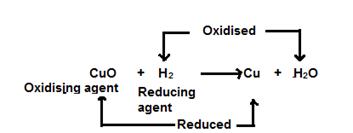
In this reaction, hydrogen acts as a reducing agent and reduces copper oxide to copper. This is a reduction reaction.
Reduction: Cu+2 + 2e−→ Cu
Simultaneously, copper oxide acts as an oxidising agent and oxidises hydrogen to water. This is an oxidation reaction.
Oxidation: 2H - 2e− → 2H+
There is one more way a reaction called redox is as follows:
Redox reactions: Redox reactions are those reactions in which both oxidation and reduction occur.
Oxidation - involves the loss of hydrogen OR gain of oxygen
Reduction - involves the gain of hydrogen OR loss of oxygen
ZnO + C → Zn +CO
In this reaction, zinc oxide is reduced to zinc and Carbon is oxidized to carbon monoxide
Oxidation - involves the loss of hydrogen OR gain of oxygen
Reduction - involves the gain of hydrogen OR loss of oxygen
ZnO + C → Zn +CO
In this reaction, zinc oxide is reduced to zinc and Carbon is oxidized to carbon monoxide
Answered by Ravi | 27 Jun, 2022, 16:17: PM
Application Videos
Concept Videos
ICSE 10 - Chemistry
Asked by jrvedant208 | 05 Feb, 2024, 22:37: PM
ICSE 10 - Chemistry
Asked by rashikulkarni28 | 10 Jul, 2022, 22:13: PM
ICSE 10 - Chemistry
Asked by rashikulkarni28 | 25 Jun, 2022, 22:24: PM
ICSE 10 - Chemistry
Asked by palshivom72 | 14 Jul, 2020, 19:56: PM
ICSE 10 - Chemistry
Asked by jhabijay01 | 27 May, 2020, 12:20: PM
ICSE 10 - Chemistry
Asked by aashimegh | 04 Sep, 2019, 08:53: AM
ICSE 10 - Chemistry
Asked by aashimegh | 04 Sep, 2019, 08:37: AM
ICSE 10 - Chemistry
Asked by aashimegh | 28 Aug, 2019, 17:25: PM