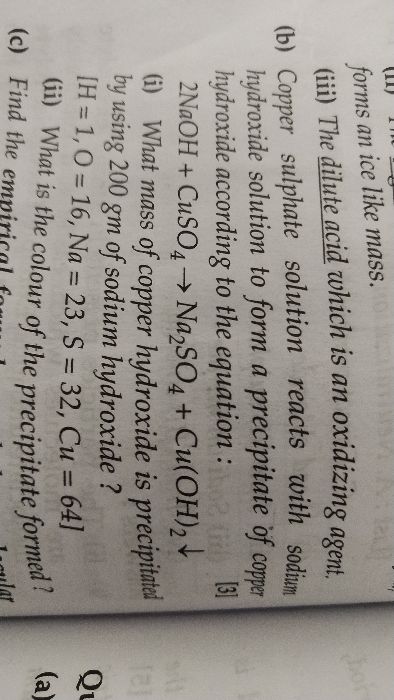ICSE Class 10 Answered
b) one ..
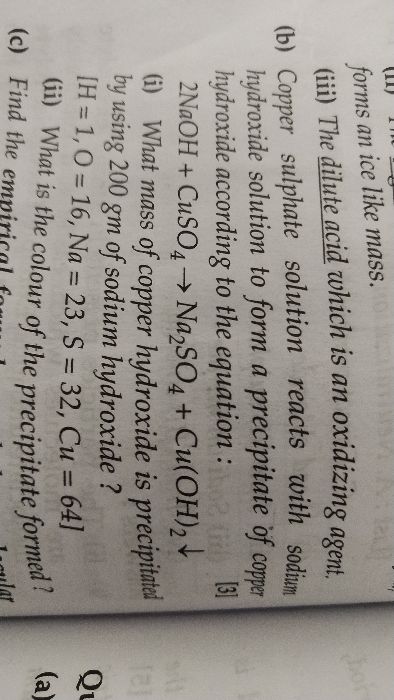
Asked by rdk_kulkarni | 09 Mar, 2021, 08:55: AM
Molecular weight of NaOH=23+16+1=98
Molecular weight of Cu(OH)2 =64+(16+1)*2=98
According to stoichiometry,
40 gram of NaOH is used to precipitate 98 g of Cu(OH)2

Answered by Ravi | 09 Mar, 2021, 22:33: PM
Application Videos
Concept Videos
ICSE 10 - Chemistry
Asked by jrvedant208 | 05 Feb, 2024, 22:37: PM
ICSE 10 - Chemistry
Asked by rashikulkarni28 | 10 Jul, 2022, 22:13: PM
ICSE 10 - Chemistry
Asked by rashikulkarni28 | 25 Jun, 2022, 22:24: PM
ICSE 10 - Chemistry
Asked by palshivom72 | 14 Jul, 2020, 19:56: PM
ICSE 10 - Chemistry
Asked by jhabijay01 | 27 May, 2020, 12:20: PM
ICSE 10 - Chemistry
Asked by aashimegh | 04 Sep, 2019, 08:53: AM
ICSE 10 - Chemistry
Asked by aashimegh | 04 Sep, 2019, 08:37: AM
ICSE 10 - Chemistry
Asked by aashimegh | 28 Aug, 2019, 17:25: PM