ICSE Class 10 Answered
Calculate the number of molecules in 1 kg of Calcium Chloride.
Asked by aashimegh | 28 Aug, 2019, 17:25: PM
Given:
Mass of calcium chloride = 1 kg
= 1000 gm
Molar mass of calcium chloride = 110.98 gm/mol
No. of moles of calcium chloride
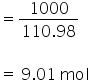
No. of molecules of CaCl2 = No. of moles of CaCl2 × Avogadro's number
= 9.01 × 6.022 × 1023
=5.42 × 1024 molecules of CaCl2
Answered by Varsha | 29 Aug, 2019, 10:28: AM
Application Videos
Concept Videos
ICSE 10 - Chemistry
Asked by jrvedant208 | 05 Feb, 2024, 22:37: PM
ICSE 10 - Chemistry
Asked by rashikulkarni28 | 10 Jul, 2022, 22:13: PM
ICSE 10 - Chemistry
Asked by rashikulkarni28 | 25 Jun, 2022, 22:24: PM
ICSE 10 - Chemistry
Asked by palshivom72 | 14 Jul, 2020, 19:56: PM
ICSE 10 - Chemistry
Asked by jhabijay01 | 27 May, 2020, 12:20: PM
ICSE 10 - Chemistry
Asked by aashimegh | 04 Sep, 2019, 08:53: AM
ICSE 10 - Chemistry
Asked by aashimegh | 04 Sep, 2019, 08:37: AM
ICSE 10 - Chemistry
Asked by aashimegh | 28 Aug, 2019, 17:25: PM