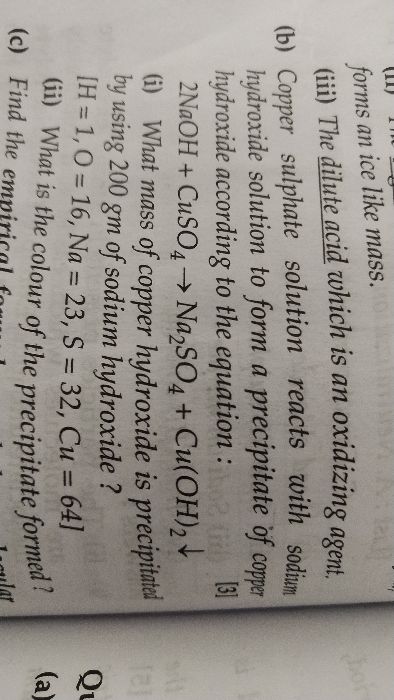ICSE Class 10 Answered
Molecular formula gives the number of atoms of each of the elements present in one molecule of a specific compound. e.g. the molecular formula of carbon dioxide is CO2
Empirical formula gives the proportions of the elements present in a compound, but not the actual numbers or arrangement of atoms is known as empirical formula, e.g., The empirical formula of benzene is CH.
Steps to determine empirical formula:
1) Write down the percentage composition and the atomic weight of each element present in the given compound.
2) Divide the percent ratio of each element by its atomic weight. The ratio gives the number of atoms of each element or relative number of atoms in the compound.
3) Select the smallest ratio amongst the relative number of atoms and divide the remaining ratios by it to give the simplest ratio of atoms present in the compound.
4) If the simplest ratio is not a whole number then multiply each ratio by the smallest suitable integer so that a whole number ratio is maintained.
5) Write the empirical formula with the atoms in the proper simple ratio of whole numbers.
Molecular formula = n(Empirical formula)
n= Molecular mass / Empirical formula mass