CBSE Class 12-science Answered
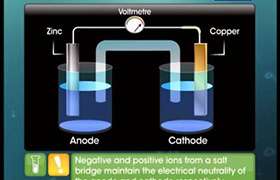
Setup the electrochemical cell for the reaction given below. 2Fe3+(aq) + Sn2+(aq)  2Fe2+(aq) + Sn4+(aq).
2Fe2+(aq) + Sn4+(aq).
Asked by Topperlearning User | 22 Jun, 2016, 02:34: PM
According to electrochemical series we find that for half cell,
F2 (g) + 2e-  2F-(aq); EO = +2.87
2F-(aq); EO = +2.87
This is the highest value of reduction potential in electrochemical series. This implies that F- cannot be oxidized by any chemical species because no element has reduction potential higher than this. F- Ion can, therefore be only electrolytic ally oxidized.
Answered by | 22 Jun, 2016, 04:34: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by summiafroz31 | 06 Feb, 2024, 08:39: PM
CBSE 12-science - Chemistry
Asked by aryamankrsinha2002 | 29 Nov, 2023, 11:39: AM
CBSE 12-science - Chemistry
Asked by banneramadevi | 26 Jul, 2023, 08:51: PM
CBSE 12-science - Chemistry
Asked by Poojanisha1988 | 19 Jul, 2023, 09:59: PM
CBSE 12-science - Chemistry
Asked by jajimuji2306 | 03 Apr, 2022, 01:38: PM
CBSE 12-science - Chemistry
Asked by Harshfarwaha | 23 Jul, 2020, 03:27: PM
CBSE 12-science - Chemistry
Asked by sourabhkumar9923 | 19 May, 2020, 08:21: PM
CBSE 12-science - Chemistry
Asked by ssharondaniel | 27 Jul, 2019, 06:22: PM
CBSE 12-science - Chemistry
Asked by kripanjalihimansu | 28 Feb, 2019, 06:57: AM
CBSE 12-science - Chemistry
Asked by govtsecschoolnayaganv051 | 12 Sep, 2018, 05:40: PM