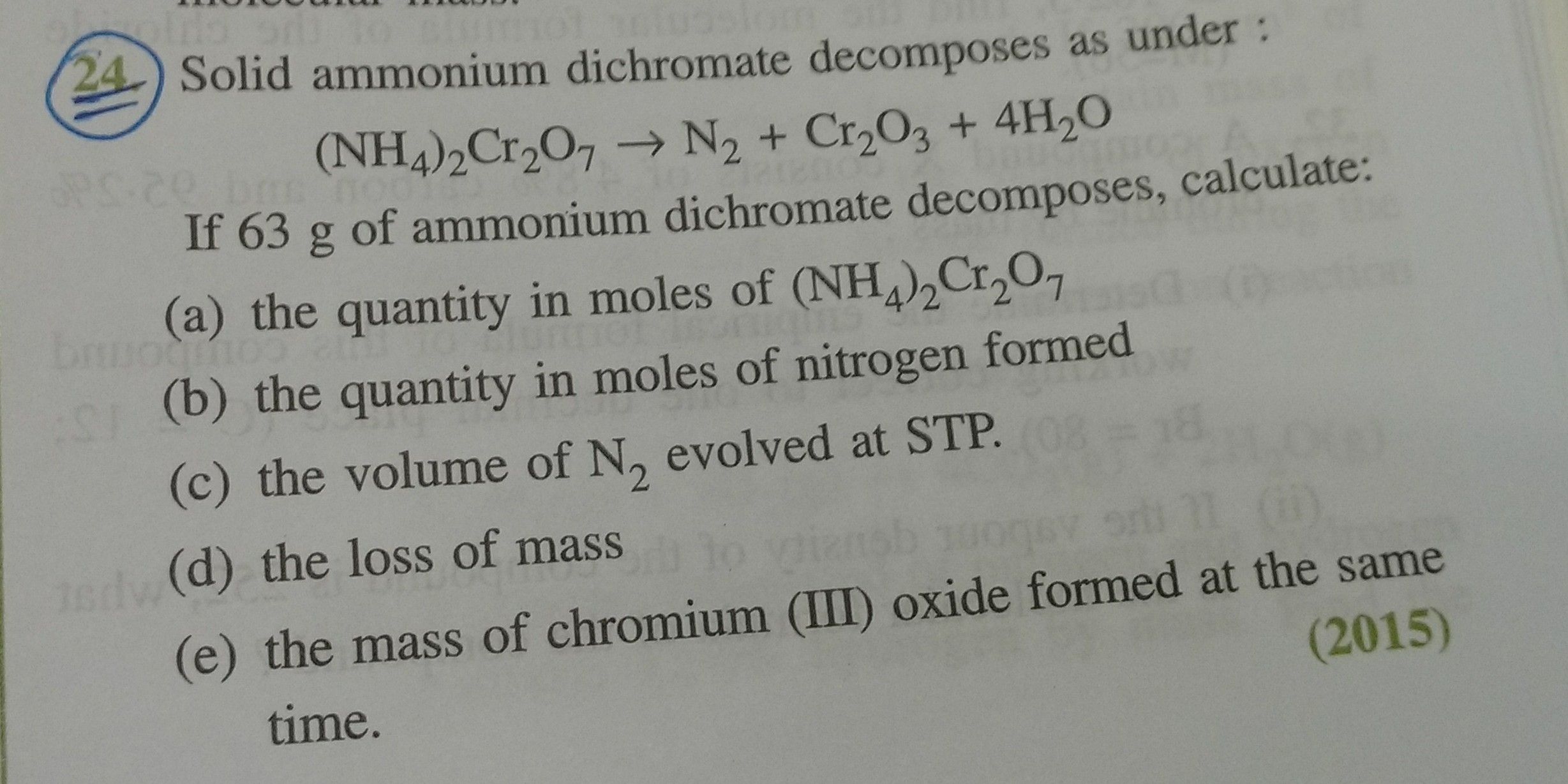ICSE Class 10 Answered
How many H2O molecules are there in a snowflake weighing 1 mg?
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
Snowflakes are made of water (H2O).The mass of 1 mole of water is calculated as follows:
Mass of H2O = 2(mass of H) + mass of O
Mass of H2O =2 (1.01g) + 16.00g
Mass of H2O = 2.02g + 16.00g
Mass of H2O = 18.02 g
One mole of H2O contains 6.022×1023 molecules of H2O (Avogadro’s number)
Therefore, 1 g of H2O contains = (6.022×1023 H2O molecules) / (18.02g)
There are 3.34×1022 H2O molecules in 1g of H2O.
So snowflake weighing 1 mg contains = (3.34×1022 molecules) / (1000)
Hence, there are 3.34×1019 H2O molecules in 1 mg snowflake.
Answered by | 04 Jun, 2014, 03:23: PM
Application Videos
Concept Videos
ICSE 10 - Chemistry
Asked by maybe.kushagra | 25 Jan, 2024, 03:12: AM
ICSE 10 - Chemistry
Asked by srinu2020.ravipati | 16 Sep, 2020, 03:33: PM
ICSE 10 - Chemistry
Asked by Gurdev71 | 24 Jun, 2020, 12:41: PM
ICSE 10 - Chemistry
Asked by Kanwaranita10 | 16 Feb, 2020, 11:22: AM
ICSE 10 - Chemistry
Asked by aashimegh | 17 Aug, 2019, 02:24: PM
ICSE 10 - Chemistry
Asked by aashimegh | 03 Aug, 2019, 11:50: AM
ICSE 10 - Chemistry
Asked by Shrinivasdangi07 | 21 Mar, 2019, 10:34: PM
ICSE 10 - Chemistry
Asked by johncena9384 | 26 Oct, 2018, 04:08: PM
ICSE 10 - Chemistry
Asked by yajay0441 | 27 Aug, 2018, 03:15: PM