ICSE Class 10 Answered
The mass of an atom of lead on=202
Asked by Shrinivasdangi07 | 21 Mar, 2019, 10:34: PM
1 mole of Pb contains Avogadro's number of atoms.
Therefore,
1 mole of Pb = 6.022 × 1023
1 mole of Pb = Molar mass of Pb
Molar mass of Pb = 202
Now,
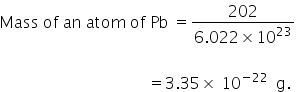
The mass of a Pb atom is 3.35 × 10−22 gm.
Answered by Varsha | 22 Mar, 2019, 12:51: PM
Application Videos
Concept Videos
ICSE 10 - Chemistry
Asked by maybe.kushagra | 25 Jan, 2024, 03:12: AM
ICSE 10 - Chemistry
Asked by srinu2020.ravipati | 16 Sep, 2020, 03:33: PM
ICSE 10 - Chemistry
Asked by Gurdev71 | 24 Jun, 2020, 12:41: PM
ICSE 10 - Chemistry
Asked by Kanwaranita10 | 16 Feb, 2020, 11:22: AM
ICSE 10 - Chemistry
Asked by aashimegh | 17 Aug, 2019, 02:24: PM
ICSE 10 - Chemistry
Asked by aashimegh | 03 Aug, 2019, 11:50: AM
ICSE 10 - Chemistry
Asked by Shrinivasdangi07 | 21 Mar, 2019, 10:34: PM
ICSE 10 - Chemistry
Asked by johncena9384 | 26 Oct, 2018, 04:08: PM
ICSE 10 - Chemistry
Asked by yajay0441 | 27 Aug, 2018, 03:15: PM