ICSE Class 10 Answered
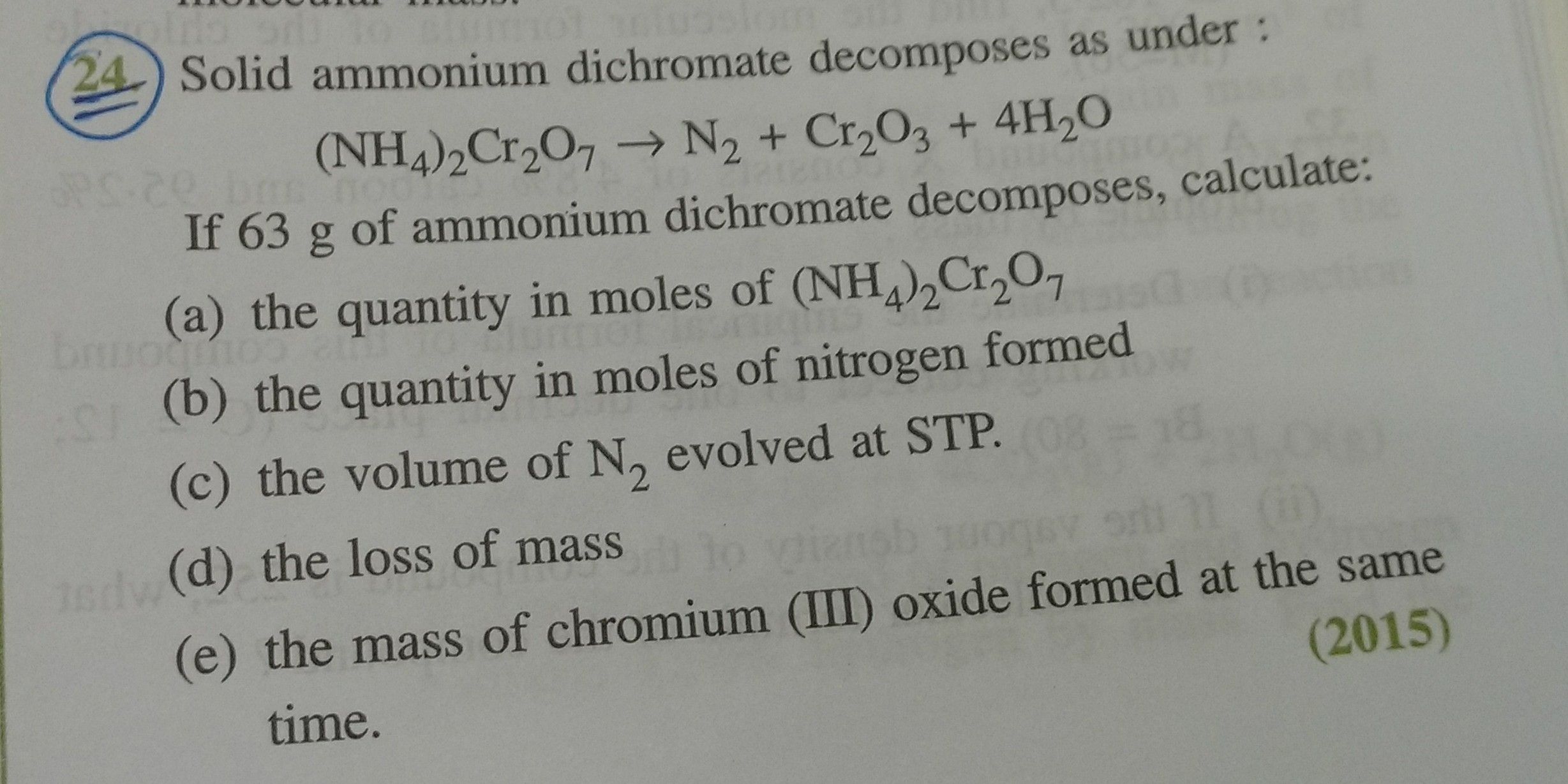
(a) The given reaction is as follows:
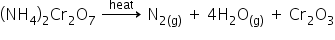
(i) Given:
Weight of (NH4)2Cr2O7 = 63 gm
Molar mass of (NH4)2Cr2O7
= (2 ×14) + (8 × 1) + (2 × 52) + (7 × 16)
= 28 + 8 + 104 + 112
= 252 gm
1 mole (NH4)2Cr2O7 = 252 gm
Hence, 63 gm of (NH4)2Cr2O7 = 0.25 moles
The quantity of moles of (NH4)2Cr2O7 if 63 gm of (NH4)2Cr2O7 is heated is 0.25 moles.
(ii) From the given chemical equation, 1 mole of (NH4)2Cr2O7 produces 1 mole of nitrogen gas.
Hence, 0.25 moles of (NH4)2Cr2O7 can produce 0.25 moles of nitrogen gas.
The quantity in moles of nitrogen formed is 0.25 moles.
(iii) One mole of an ideal gas at S.T.P. occupies 22.4 litres or dm3.
Hence, 0.25 moles of (NH4)2Cr2O7 will occupy 0.25 ´ 22.4 = 5.6 litres or dm3.
The volume in litres or dm3 of N2 evolved at S.T.P. is 5.6 litres or dm3.
(iv) From the given chemical equation, 1 mole of (NH4)2Cr2O7 produces 1 mole of Cr2O3.
Hence, 0.25 moles of (NH4)2Cr2O7 will produce 0.25 moles of Cr2O3.
Molar mass of Cr2O3 = (2 × 52) + (3 × 16)
= 104 + 48
= 152 gm
(v) 1 mole Cr2O3 = 152 gm
Hence, 0.25 moles of Cr2O3 = 0.25 × 152 = 38 gm
The mass in grams of Cr2O3 formed at the same time is 38 gm.








