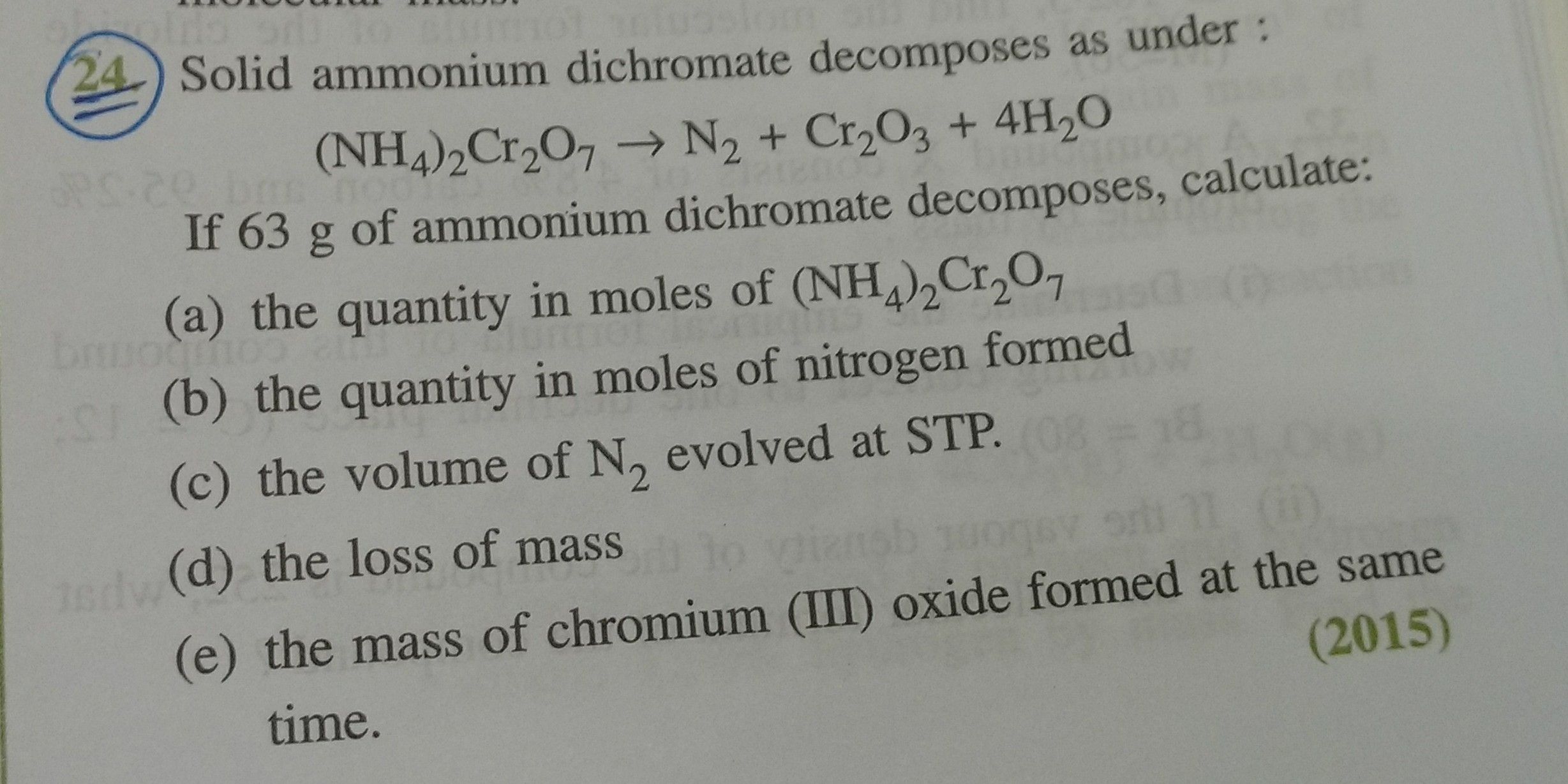ICSE Class 10 Answered
Calculate the relative molecular mass[molecular weight] of 290 ml of a gas A at 17 degrees celsius and 1520 mm pressure which weighs 2.73 gram at S.T.P.
Asked by johncena9384 | 26 Oct, 2018, 04:08: PM
Given:
P =1520 mm
V = 90 ml
Weight = 2.73 gm
T = 17 °C
= 300 K
We have,
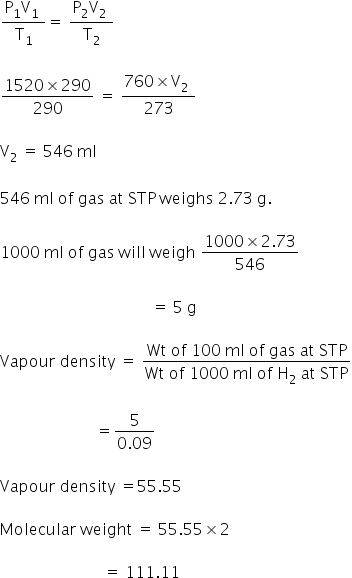
molecular weight of gas is 111.11
Answered by Varsha | 29 Oct, 2018, 06:15: PM
Application Videos
Concept Videos
ICSE 10 - Chemistry
Asked by maybe.kushagra | 25 Jan, 2024, 03:12: AM
ICSE 10 - Chemistry
Asked by srinu2020.ravipati | 16 Sep, 2020, 03:33: PM
ICSE 10 - Chemistry
Asked by Gurdev71 | 24 Jun, 2020, 12:41: PM
ICSE 10 - Chemistry
Asked by Kanwaranita10 | 16 Feb, 2020, 11:22: AM
ICSE 10 - Chemistry
Asked by aashimegh | 17 Aug, 2019, 02:24: PM
ICSE 10 - Chemistry
Asked by aashimegh | 03 Aug, 2019, 11:50: AM
ICSE 10 - Chemistry
Asked by Shrinivasdangi07 | 21 Mar, 2019, 10:34: PM
ICSE 10 - Chemistry
Asked by johncena9384 | 26 Oct, 2018, 04:08: PM
ICSE 10 - Chemistry
Asked by yajay0441 | 27 Aug, 2018, 03:15: PM