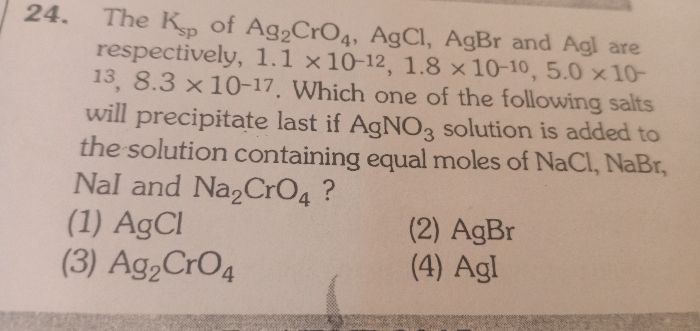CBSE Class 11-science Answered
explain dissolution of sodium chloride in water.
Asked by ABHILASHA | 23 Oct, 2019, 10:44: PM
Water is a olar solvent so polarity of water molecules enables water to dissolve ionic substance. Sodium chloride is a salt which is an ionic compound. It is made up of sodium(poitive ion) and chloride(negative ion). Water can dissolve salt because the positive part of water molecules attracts the negative chloride and negative part of water molecules attracts postive sodium. These positive and negative ions are pulled apart by water molecules.
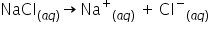
Answered by Ravi | 24 Oct, 2019, 03:31: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by achamerahul2 | 21 Apr, 2020, 02:33: PM
CBSE 11-science - Chemistry
Asked by achamerahul2 | 21 Apr, 2020, 02:32: PM
CBSE 11-science - Chemistry
Asked by mufeedatvp2000 | 18 Apr, 2020, 02:21: PM
CBSE 11-science - Chemistry
Asked by Anish | 23 Aug, 2019, 01:48: AM
CBSE 11-science - Chemistry
Asked by jhajuhi19 | 12 Jun, 2019, 07:23: PM
CBSE 11-science - Chemistry
Asked by Prakash | 28 Jun, 2018, 06:09: PM
CBSE 11-science - Chemistry
Asked by gganga | 10 Apr, 2018, 06:31: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 May, 2015, 03:13: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 30 Apr, 2015, 02:30: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 16 Jun, 2016, 05:24: PM