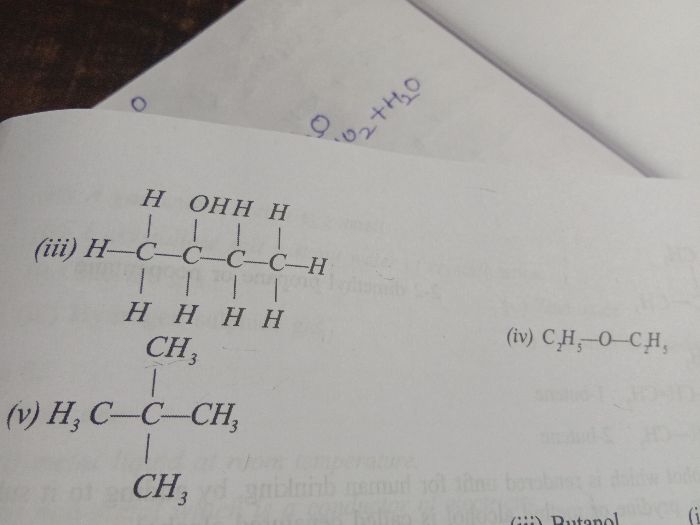ICSE Class 10 Answered
Differentiate saturated and unsaturated hydrocarbons.
Why are they called so in this manner
Asked by lovemaan5500 | 29 Oct, 2017, 10:42: AM
| Saturated hydrocarbons | Unsaturated hydrocarbons |
|
These are the organic compound with single covalent
bond.
|
These are the organic compound with carbon- carbon double or triple
bond.
|
| These are less reactive. | These are more reactive. |
| These includes alkanes. | These includes alkenes and alkynes. |
| They have more hydrogen atoms bonded to carbon atoms. | They have fewer hydrogen atoms bonded to carbon atom. |
Saturated hydrocarbons
These are the hydrocarbons in which carbon atoms are bonded to aother atoms by single covalent bonds.Therefore they do not contain multiple bonds that is double bond or triple bonds.
Hence all carbon atoms are fully ocuppied by forming four bonds.Therefore they are called as saturated compunds.
Unsaturated hydrocarbons
These are the hydrocarbons with carbon - carbon double or triple bond.They are called unsaturated as carbon atoms do not have as many hydrogen atoms as they possibly could.
Answered by Varsha | 30 Oct, 2017, 11:57: AM
Application Videos
Concept Videos
ICSE 10 - Chemistry
Asked by anshpatel6307 | 18 Mar, 2022, 09:32: PM
ICSE 10 - Chemistry
Asked by pritijpjain | 13 Feb, 2021, 07:44: AM
ICSE 10 - Chemistry
Asked by Kanwaranita10 | 16 Feb, 2020, 09:23: AM
ICSE 10 - Chemistry
Asked by arpitt682 | 01 Oct, 2019, 03:44: PM
ICSE 10 - Chemistry
Asked by vijay.prag | 14 Jul, 2019, 09:35: AM
ICSE 10 - Chemistry
Asked by jaiswalsindhuli717 | 16 Dec, 2018, 05:20: PM
ICSE 10 - Chemistry
Asked by hy666333 | 12 Dec, 2018, 08:04: PM
ICSE 10 - Chemistry
Asked by jaiswalsindhuli717 | 12 Nov, 2018, 08:29: PM
ICSE 10 - Chemistry
Asked by jaiswalsindhuli717 | 12 Nov, 2018, 08:18: PM
ICSE 10 - Chemistry
Asked by dr_pradip27121972 | 03 Jun, 2018, 12:55: PM