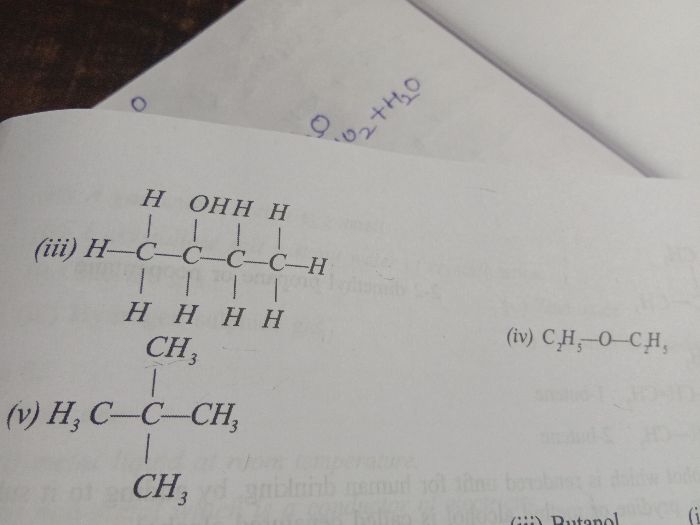ICSE Class 10 Answered
According to the IUPAC system, the name of an organic compound consists of three parts:
- Root word
- Suffix
- Prefix
Root Word:
It depends on the number of carbon atoms present in the longest carbon chain selected.
|
Number of carbon atoms |
Root word |
|
One carbon atom C1 |
Meth |
|
Two carbon atoms C2 |
Eth |
|
Three carbon atoms C3 |
Prop |
|
Four carbon atoms C4 |
But |
|
Five carbon atoms C5 |
Pent |
|
Six carbon atoms C6 |
Hex |
|
Seven carbon atoms C7 |
Hept |
|
Eight carbon atoms C8 |
Oct |
|
Nine carbon atoms C9 |
Non |
|
Ten carbon atoms C10 |
Dec |
Suffix:
The root word is followed by an appropriate suffix which represents the nature of the bond in a carbon–carbon atom.
|
Nature of bond |
Suffix |
General name |
General formula |
|
Single bond (C–C) |
-ane |
Alkane |
CnH2n+2 |
|
Double bond (C=C) |
-ene |
Alkene |
CnH2n |
|
Triple bond ( ) |
-yne |
Alkyne |
CnH2n−2 |
|
Group (R-) |
-yl |
Alkyl |
CnH2n+1 |
Prefix:
It denotes the substituent, alkyl or functional group and its position in the carbon chain.
Di-, tri- and tetra- are used for two, three and four groups of the same type, respectively.
In naming an organic compound, the following simple rules are followed:
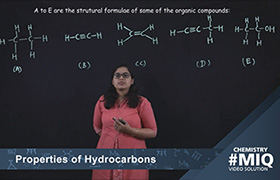
1. Selection of carbon chains: The longest continuous chain of ‘C’ atoms, known as parent chain, is selected. The longest chain need not be straight. For example:
Here, the longest chain is of 7 carbon atoms, so the root word is ‘hept’, the remaining carbon atoms are substituents.
2. The branch chains are considered substituents, and their positions are indicated by the number of carbon atoms to which they are attached.
For example:
3. The carbon atoms of the longest chain are numbered in such a way that the alkyl groups get the smallest possible number.
For example:
4. In case, any functional group is present in the chain, the carbon atoms are numbered in such a way that the functional group gets the smallest possible number.
For example:
5. In case, different types of substituents are attached to the chain, they are arranged and named alphabetically.
2,Bromo,4 chloro hexane
6. The positions of alkyl groups are indicated by writing the position and name of the alkyl group just before the name of the parent hydrocarbon.
3-ethylheptane
7. Multiple alkyl groups are labeled with the Greek numerical prefixes such as ‘di’ for two, ‘tri’ for three, ‘tetra’ for four, ‘penta’ for five.
If two alkyl groups are on the same carbon atom, then the numeral is repeated.