CBSE Class 11-science Answered
Q1 making use of the concept of hybridisation ,discuss the shape of:
BeCl2,BF3 and Ch4 molecules
Q2 draw shapesof sp , sp2, and sp3 hybrid orbitals?
Q3 give 4 difference between sigma and pi bond?
Asked by rajenderdagar123 | 08 Jan, 2017, 06:50: PM
Dear rajenderdagar123@gmail.com
Thanks for asking us a question in Ask the Expert section of TopperLearning.com.
We cannot entertain more than 3 questions in a single query per day. In case of multiple questions within a query, please post each question individually and let us know where you are getting stuck so that we would be able to explain things better.
Answer to your first question is given below:
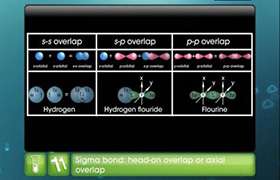
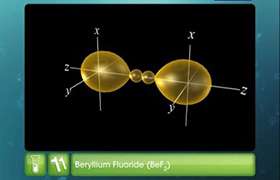
Structure of BeCl2:
- In BeCl2, the two singly occupied orbitals (2s and 2p) hybridise to give two sp-hybrid orbitals. These hybrid orbitals lie along the z-direction and point in opposite directions.
- The ground state electronic configuration of Be is 1s22s2. In the exited state, one of the 2s-electrons is promoted to the vacant 2p orbital to account for its bivalency.
- One 2s and one 2p-orbital hybridise to form two sp hybridised orbitals. These two sp hybrid orbitals are oriented in opposite directions forming an angle of 180°.
Answered by Vaibhav Chavan | 09 Jan, 2017, 10:59: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by Trisha Gupta | 30 Oct, 2022, 05:36: PM
CBSE 11-science - Chemistry
Asked by ABHILASHA | 22 Aug, 2020, 04:39: AM
CBSE 11-science - Chemistry
Asked by kpbhake | 12 Mar, 2018, 11:45: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 08 Oct, 2014, 01:09: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 13 Jun, 2016, 02:26: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Chemistry
What is the hybrid state of B in BF3, Al in AlCl3, Be in BeCl2, C in CO2 and C2H4; S in SO2 and SO3.
Asked by Topperlearning User | 08 Oct, 2014, 01:33: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 09 Oct, 2014, 09:30: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM


 on the basis of hybridisation
on the basis of hybridisation