CBSE Class 11-science Answered
Differentiate between valence bond theory and Lewis concept with regard to the formation of covalent bond.
Asked by Topperlearning User | 09 Oct, 2014, 09:30: AM
|
Lewis concept
|
Valence bond theory
|
|
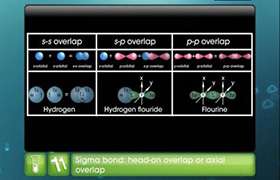
i) Lewis concept considers the formation of covalent bond by mutual sharing of electrons.
|
i) Valence bond theory considers the formation of covalent bond by overlap of half filled atomic orbitals.
|
|
ii) It does not provide explanation for different shape of molecules.
|
ii) Valence bond theory explains the shape of molecules.
|
|
iii) Lewis structure does not explain the bond strength.
|
iii) Valence bond theory explains the bond strength.
|
Answered by | 09 Oct, 2014, 11:30: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by Trisha Gupta | 30 Oct, 2022, 05:36: PM
CBSE 11-science - Chemistry
Asked by ABHILASHA | 22 Aug, 2020, 04:39: AM
CBSE 11-science - Chemistry
Asked by kpbhake | 12 Mar, 2018, 11:45: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 08 Oct, 2014, 01:09: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 13 Jun, 2016, 02:26: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Chemistry
What is the hybrid state of B in BF3, Al in AlCl3, Be in BeCl2, C in CO2 and C2H4; S in SO2 and SO3.
Asked by Topperlearning User | 08 Oct, 2014, 01:33: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 09 Oct, 2014, 09:30: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM


 on the basis of hybridisation
on the basis of hybridisation