CBSE Class 11-science Answered
How many lone pairs, bond pairs are present around S in SF4 molecule? What is their arrangement?
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
In SF4 molecule, 4 bond pair and 1 lone pair is present. The arrangement is trigonal bipyramidal.
Answered by | 04 Jun, 2014, 03:23: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by Trisha Gupta | 30 Oct, 2022, 05:36: PM
CBSE 11-science - Chemistry
Asked by ABHILASHA | 22 Aug, 2020, 04:39: AM
CBSE 11-science - Chemistry
Asked by kpbhake | 12 Mar, 2018, 11:45: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 08 Oct, 2014, 01:09: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 13 Jun, 2016, 02:26: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Chemistry
What is the hybrid state of B in BF3, Al in AlCl3, Be in BeCl2, C in CO2 and C2H4; S in SO2 and SO3.
Asked by Topperlearning User | 08 Oct, 2014, 01:33: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 09 Oct, 2014, 09:30: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM


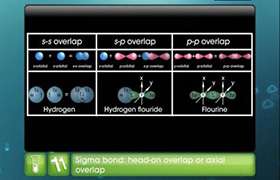
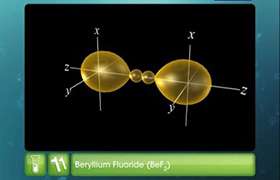
 on the basis of hybridisation
on the basis of hybridisation