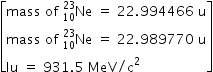CBSE Class 12-science Physics Nuclear Energy
-
nuclei
-
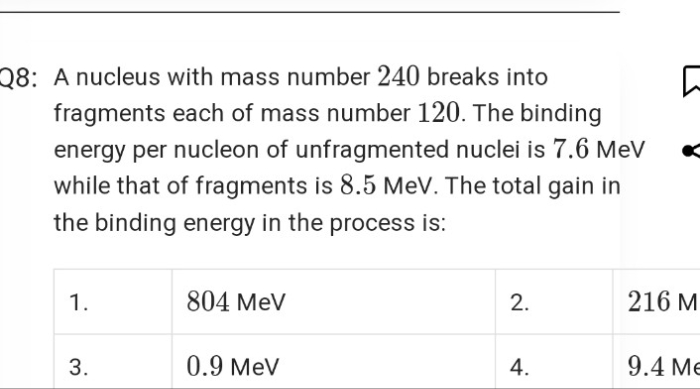
. A nucleus of mass number 240 and having binding energy/nucleon 7.6 MeV splits into two fragments Y, Z of mass numbers 110 and 130 respectively. If the binding energy/nucleon of Y, Z is equal to 8.5 MeV each, calculate the energy released in the nuclear reaction.

- Please answer this question. Draw a graph showing the variation of binding energy per nucleon as a function of mass number A. The binding energy per nucleon for heavy nuclei (A > 170) decreases with the increase in mass number. Explain.
- Binding energies of 8O16 and 17Cl35 are 127.35 MeV and 289.3 MeV respectively. Which of the two nuclei are more stable?
- What is Q- value of a nuclear reaction?
-
A neutron is absorbed by a 3 L
 nucleus with subsequent emission of
nucleus with subsequent emission of  - particle Write the corresponding nuclear reaction. Calculate the energy released in this reaction.
Given mass of 3 L
- particle Write the corresponding nuclear reaction. Calculate the energy released in this reaction.
Given mass of 3 L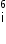 = 6.015126 a.m.u.
Mass of 2
= 6.015126 a.m.u.
Mass of 2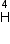 e = 4.000 26044 a.m.u.
Mass of neutron
e = 4.000 26044 a.m.u.
Mass of neutron 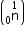 = 1.0086654 a.m.u.
Mass of tritium
= 1.0086654 a.m.u.
Mass of tritium 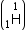 = 3.016049 a.m.u.
= 3.016049 a.m.u.
- Why is nuclear fusion not possible in laboratory?
- Express 16mg mass into equivalent energy in electron volt?
- Draw a curve between mass number and binding energy per nucleon. Give two salient features of the curve. Hence define binding energy?
-
A nucleus
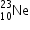 undergoes β- decay and becomes
undergoes β- decay and becomes 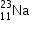 , Calculate the maximum kinetic energy of electrons emitted assuming that the daughter nucleus and anti- neutrino carry negligible kinetic energy.
, Calculate the maximum kinetic energy of electrons emitted assuming that the daughter nucleus and anti- neutrino carry negligible kinetic energy.