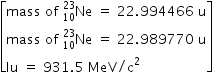CBSE Class 12-science Answered
A neutron is absorbed by a 3 L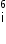 nucleus with subsequent emission of
nucleus with subsequent emission of 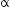 - particle Write the corresponding nuclear reaction. Calculate the energy released in this reaction.
Given mass of 3 L
- particle Write the corresponding nuclear reaction. Calculate the energy released in this reaction.
Given mass of 3 L = 6.015126 a.m.u.
Mass of 2
= 6.015126 a.m.u.
Mass of 2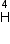 e = 4.000 26044 a.m.u.
Mass of neutron
e = 4.000 26044 a.m.u.
Mass of neutron 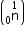 = 1.0086654 a.m.u.
Mass of tritium
= 1.0086654 a.m.u.
Mass of tritium 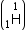 = 3.016049 a.m.u.
= 3.016049 a.m.u.
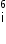 nucleus with subsequent emission of
nucleus with subsequent emission of 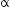 - particle Write the corresponding nuclear reaction. Calculate the energy released in this reaction.
Given mass of 3 L
- particle Write the corresponding nuclear reaction. Calculate the energy released in this reaction.
Given mass of 3 L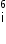 = 6.015126 a.m.u.
Mass of 2
= 6.015126 a.m.u.
Mass of 2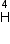 e = 4.000 26044 a.m.u.
Mass of neutron
e = 4.000 26044 a.m.u.
Mass of neutron 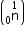 = 1.0086654 a.m.u.
Mass of tritium
= 1.0086654 a.m.u.
Mass of tritium 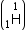 = 3.016049 a.m.u.
= 3.016049 a.m.u.
Asked by Topperlearning User | 02 Jun, 2015, 10:23: AM
Nuclear reaction is given by
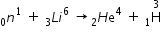
Mass of reactants = m 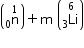
= 1.0086654 + 6.015126 = 7.0237914 a.m.u.
Mass Defect, Δm = mass of reactant - mass of product.
Δm = 7.0237194 - 7.0186534
Δm = 0.005138 a.m.u.
Since1. a.m.u. = 931 MeV
 Energy released =Δm x 931 MeV
Energy released =Δm x 931 MeV
E = 0.005138 x 931
| E = 4.783MeV |
Answered by | 02 Jun, 2015, 12:23: PM
CBSE 12-science - Physics
Asked by murshidibrahimkk | 08 Feb, 2024, 10:28: AM
CBSE 12-science - Physics
Asked by kailasks2007 | 28 Dec, 2023, 20:14: PM
CBSE 12-science - Physics
Asked by varma.renu9481 | 06 Mar, 2023, 17:44: PM
CBSE 12-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Physics
Asked by Topperlearning User | 02 Jun, 2015, 13:03: PM
CBSE 12-science - Physics
Asked by Topperlearning User | 02 Jun, 2015, 10:23: AM
CBSE 12-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Physics
Asked by Topperlearning User | 09 Jul, 2014, 12:48: PM


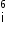 nucleus with subsequent emission of
nucleus with subsequent emission of 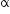 - particle Write the corresponding nuclear reaction. Calculate the energy released in this reaction.
Given mass of 3 L
- particle Write the corresponding nuclear reaction. Calculate the energy released in this reaction.
Given mass of 3 L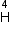 e = 4.000 26044 a.m.u.
Mass of neutron
e = 4.000 26044 a.m.u.
Mass of neutron 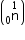 = 1.0086654 a.m.u.
Mass of tritium
= 1.0086654 a.m.u.
Mass of tritium 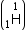 = 3.016049 a.m.u.
= 3.016049 a.m.u.
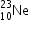 undergoes β- decay and becomes
undergoes β- decay and becomes 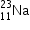 , Calculate the maximum kinetic energy of electrons emitted assuming that the daughter nucleus and anti- neutrino carry negligible kinetic energy.
, Calculate the maximum kinetic energy of electrons emitted assuming that the daughter nucleus and anti- neutrino carry negligible kinetic energy.