CBSE Class 12-science Answered
Please answer this question.
Draw a graph showing the variation of binding energy per nucleon as a function of mass number A. The binding energy per nucleon for heavy nuclei (A > 170) decreases with the increase in mass number. Explain.
Asked by varma.renu9481 | 06 Mar, 2023, 17:44: PM
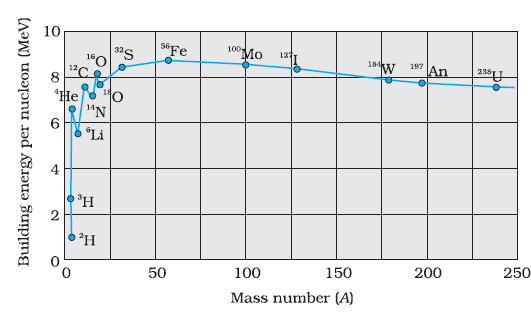
Biding energy per nucleon decreases after mass number A > 170 because for the elements A > 170 ,
number of neutrons in the nuclei is greater than number of protons .
For example , Tungsten (W) ,
Mass number A = 184 ,Atomic number Z = 74 , Hence Tungsten (W) element has 74 protons and 110 neutrons
For example , Gold (Au)
Mass number A = 197, atomic number z = 79 . Hence Gold (Au) has 79 protons and 118 neutrons.
Greater number of neutrons than protons makes less binding energy to the nucleus .
Hence as neutron number inreases , binding energy per nucleon decreases.
Answered by Thiyagarajan K | 06 Mar, 2023, 23:32: PM
CBSE 12-science - Physics
Asked by murshidibrahimkk | 08 Feb, 2024, 10:28: AM
CBSE 12-science - Physics
Asked by kailasks2007 | 28 Dec, 2023, 20:14: PM
CBSE 12-science - Physics
Asked by varma.renu9481 | 06 Mar, 2023, 17:44: PM
CBSE 12-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Physics
Asked by Topperlearning User | 02 Jun, 2015, 13:03: PM
CBSE 12-science - Physics
Asked by Topperlearning User | 02 Jun, 2015, 10:23: AM
CBSE 12-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Physics
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 12-science - Physics
Asked by Topperlearning User | 09 Jul, 2014, 12:48: PM


 nucleus with subsequent emission of
nucleus with subsequent emission of 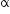 - particle Write the corresponding nuclear reaction. Calculate the energy released in this reaction.
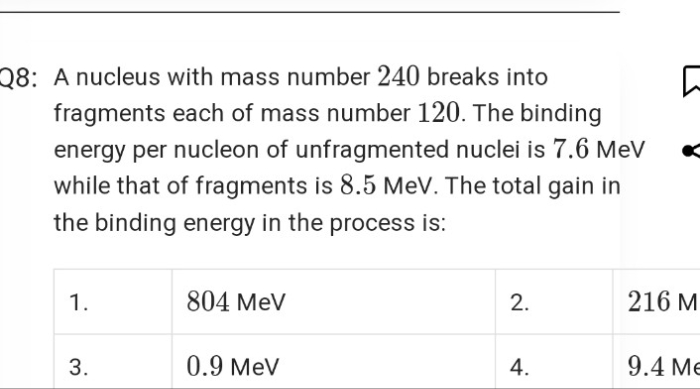
Given mass of 3 L
- particle Write the corresponding nuclear reaction. Calculate the energy released in this reaction.
Given mass of 3 L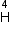 e = 4.000 26044 a.m.u.
Mass of neutron
e = 4.000 26044 a.m.u.
Mass of neutron 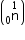 = 1.0086654 a.m.u.
Mass of tritium
= 1.0086654 a.m.u.
Mass of tritium 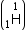 = 3.016049 a.m.u.
= 3.016049 a.m.u.
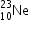 undergoes β- decay and becomes
undergoes β- decay and becomes 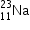 , Calculate the maximum kinetic energy of electrons emitted assuming that the daughter nucleus and anti- neutrino carry negligible kinetic energy.
, Calculate the maximum kinetic energy of electrons emitted assuming that the daughter nucleus and anti- neutrino carry negligible kinetic energy.