CBSE Class 11-science - Redox Reactions Videos
Redox Reactions
This video explains oxidation, reduction in terms of classical view and in terms of electron loss or gain.
More videos from this chapter
View All- what is acid
- explain redox
-
pls explain
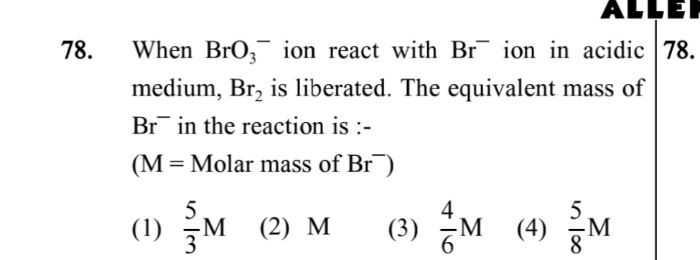
-
- For the reaction, MnO4?+ C2O42? ? MnO2+ CO2 ; The simplest mole ratio of MnO4? and C2O42? in which they react is
- It takes 0.15 mole of ClO- to oxidize 12.6g of chromium oxide of a specific formula to Cr2O7 ^ 2-.ClO- became Cl-.The formula of the oxide is atomic weight Cr- 52, O- 16) (a)CrO3 (b)CrO2 (c)CrO4 (d)CrO
- What is the path of a redox reaction? In photosynthesis by using H2O(18) {O-18 isotope in H2O} how can we find path of reaction? Please help
- NaH+H2O=NaOH+ H2 which type of redox reaction is this? Pls explain in detail
- 5g of pyrolusite were heated with conc. HCl and Cl2 evolved was passed through excess of KI solution. The iodine liberated required 40 mL of N/10hypo solution.Find the % of MnO2 in the pyrolusite.
- P4+OHminus gives PH3H2PO2 redox reaction







