CBSE Class 11-science Answered
For the reaction, MnO4?+ C2O42? ? MnO2+ CO2 ; The simplest mole ratio of MnO4? and C2O42? in which they react is
Asked by debjit_dm | 04 May, 2020, 15:08: PM
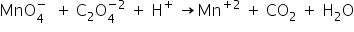
Oxidation number of Mn in 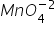
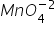
x-8=-1
x=+7
Change of oxidation number of Mn is from +7 to +2
The decrease in oxidation number is=7-2=5
Oxidation number of C increases from +3 to +4.
The change in oxidation number for 1 C atom=4-3=1
The increase in oxidation number for 2 C atom=2
1) To balance the oxidation number-
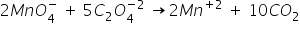
2) Balance the O atoms by adding 8 water molecules on product side-
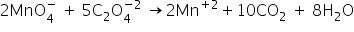
3) Balance the H atoms by adding 16 H+ ions to reactant side-
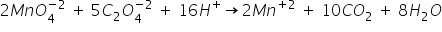
This is the balanced equation.
The simples molar ratio of these reactants is 2:5.
Answered by Ravi | 04 May, 2020, 19:00: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by patrasudipkumar191 | 05 Jul, 2024, 09:36: AM
CBSE 11-science - Chemistry
Asked by jaip83491 | 24 Jan, 2021, 10:43: AM
CBSE 11-science - Chemistry
Asked by sulaikhasulu393 | 07 Jun, 2020, 22:51: PM
CBSE 11-science - Chemistry
Asked by debjit_dm | 04 May, 2020, 15:08: PM
CBSE 11-science - Chemistry
Asked by Balbir | 30 Jun, 2019, 15:45: PM
CBSE 11-science - Chemistry
Asked by govtsecschoolnayaganv051 | 05 Sep, 2018, 13:32: PM
CBSE 11-science - Chemistry
Asked by govtsecschoolnayaganv051 | 09 Aug, 2018, 16:52: PM
CBSE 11-science - Chemistry
Asked by dineshchem108 | 17 Jul, 2018, 18:02: PM
CBSE 11-science - Chemistry
Asked by vaagai2353 | 29 Jun, 2018, 21:55: PM






