CBSE Class 11-science Answered
It takes 0.15 mole of ClO- to oxidize 12.6g of chromium oxide of a specific formula to Cr2O7 ^ 2-.ClO- became Cl-.The formula of the oxide is atomic weight Cr- 52, O- 16)
(a)CrO3
(b)CrO2
(c)CrO4
(d)CrO
Asked by Balbir | 30 Jun, 2019, 15:45: PM
Let's suppose formula of Chromium oxide is CrxOy
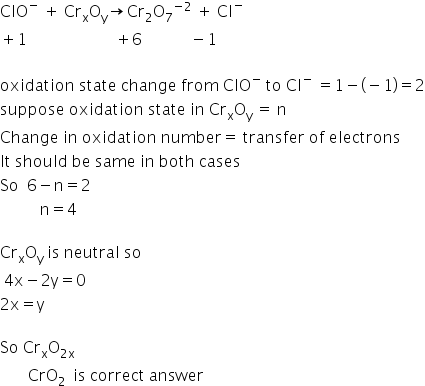
Answered by Ravi | 01 Jul, 2019, 14:31: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by patrasudipkumar191 | 05 Jul, 2024, 09:36: AM
CBSE 11-science - Chemistry
Asked by jaip83491 | 24 Jan, 2021, 10:43: AM
CBSE 11-science - Chemistry
Asked by sulaikhasulu393 | 07 Jun, 2020, 22:51: PM
CBSE 11-science - Chemistry
Asked by debjit_dm | 04 May, 2020, 15:08: PM
CBSE 11-science - Chemistry
Asked by Balbir | 30 Jun, 2019, 15:45: PM
CBSE 11-science - Chemistry
Asked by govtsecschoolnayaganv051 | 05 Sep, 2018, 13:32: PM
CBSE 11-science - Chemistry
Asked by govtsecschoolnayaganv051 | 09 Aug, 2018, 16:52: PM
CBSE 11-science - Chemistry
Asked by dineshchem108 | 17 Jul, 2018, 18:02: PM
CBSE 11-science - Chemistry
Asked by vaagai2353 | 29 Jun, 2018, 21:55: PM






