CBSE Class 11-science - Ionisation of Water Videos
Equilibrium
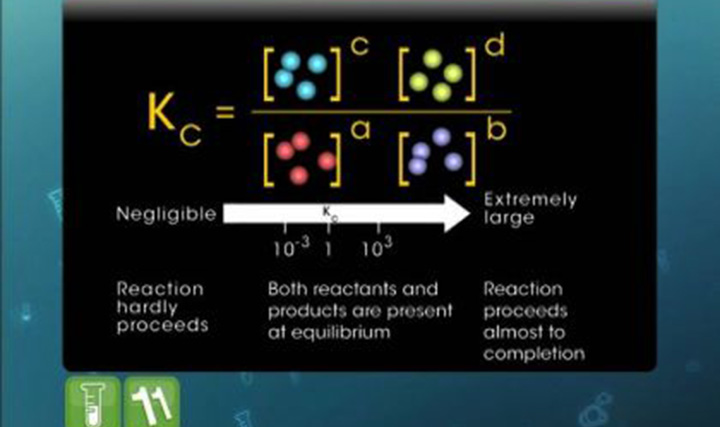
This video explains ionisation of water, ionic products of water and the dissociation constant of water.
More videos from this chapter
View All- Define the term salt hydrolysis?
- Discuss different types of salt hydrolysis.
- The pH of lemon juice is 2.32 at 298 k. What will be its [H3O+]?
- A solution is made by dissolving 0.63 gm of nitric acid in 200 ml of water, assuming that the acid is completely dissociated, calculate its pH value.
- The pH of a soil sample is 8.3. What is the value of [HO-] of the sample?
- Calculate the pH of a solution that has [HO-] concentration of 0.0026 mol/L.
-
Calculate the value of Ka for the following reaction having the equilibrium concentration of H+ 7.1 x 10-5 mol/L in a 0.05 molar solution. H2S
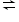 H+ + HS-
H+ + HS-
- Calculate the pH of a solution that has a [OH1-] = 1 X 10-5 M
- Define degree of hydrolysis (h).
- Explain ionic product of water. What is the effect of temperature on Ionic product of water?