CBSE Class 11-science Answered
Explain ionic product of water. What is the effect of temperature on Ionic product of water?
Asked by Topperlearning User | 16 Jun, 2016, 17:21: PM
Water is a weak electrolyte and ionizes to a very small extent forming [H+]
and [HO-] as follows.
H2O 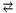 H+ + HO-
H+ + HO-
Whereby, 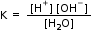
Or, K. [H2O] = [H+] [HO-]
Or, Kw = [H+] [HO-]
Where Kw is known as ionic product of water. The value of Kw at 298 k is about 1.0x 10-14 M2.
The value of Kw changes with temperature.
Answered by | 16 Jun, 2016, 19:21: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by Topperlearning User | 16 Jun, 2016, 17:22: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 29 Apr, 2015, 14:41: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 30 Apr, 2015, 08:49: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 30 Apr, 2015, 09:57: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 30 Apr, 2015, 10:51: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 30 Apr, 2015, 11:04: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 30 Apr, 2015, 11:08: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 16 Jun, 2016, 17:21: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 16 Jun, 2016, 17:21: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 16 Jun, 2016, 17:21: PM




 H+ + HS-
H+ + HS-