CBSE Class 11-science Answered
The pH of a soil sample is 8.3. What is the value of [HO-] of the sample?
Asked by Topperlearning User | 30 Apr, 2015, 10:51: AM
pH = -log[H3O+]
1/ [H3O+] = antilog pH
antilog 8.3 = 6.76x 108
or, [H3O+] = 1/6.76x 108 = 1.47 x 10-9 M
[HO-] = Kw / [H+] = 10-14/1.47 x 10-9 M = 6.76 x 10-6 M
Answered by | 30 Apr, 2015, 12:51: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by Topperlearning User | 16 Jun, 2016, 17:22: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 29 Apr, 2015, 14:41: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 30 Apr, 2015, 08:49: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 30 Apr, 2015, 09:57: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 30 Apr, 2015, 10:51: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 30 Apr, 2015, 11:04: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 30 Apr, 2015, 11:08: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 16 Jun, 2016, 17:21: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 16 Jun, 2016, 17:21: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 16 Jun, 2016, 17:21: PM




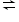 H+ + HS-
H+ + HS-