CBSE Class 10 - Chemical Equations Videos
Chemical Equation - Part 2
This video explains how to write chemical equations and represent chemical reactions.
More videos from this chapter
View All- what was the formula of water
- how to solve valency
- what is chemical formula
- write the chemical equation for reaction that takes place when lead nitrate and potassium iodine solutions are mixed
- C5H8O2+ NaH+ HCl→____C5H12O2+ NaCl balancing the equation
-
Balance the following equation—
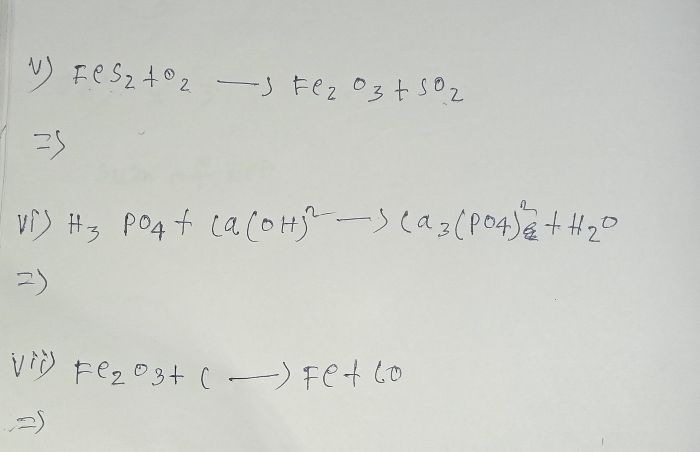
- On what basis is a chemical equation balanced?
- what happen when quicklime is added to water
- compound name of the formule KNO
- NaCl+H2O=NaOH+H2+Cl2






