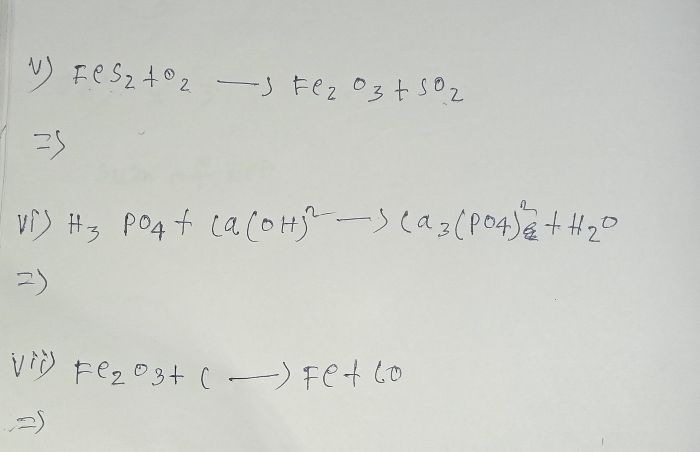CBSE Class 10 Answered
how to solve valency
Asked by yadavparmit83 | 01 Dec, 2023, 06:16: AM
Dear Student,
Valency is the combining capacity of an atom or a radical.
For example, valency of carbon is 4 because it combines with four atoms of hydrogen to yield methane (CH4).
Valency with Respect to Hydrogen Atom The number of hydrogen atoms that combines with or displaces one atom of that element or radical.
The valency is taken to be 1 and is considered as standard.
Modern Definition of Valency: The number of electrons which an atom can lose, gain or share during a chemical reaction to attain the stable configuration of the nearest inert gas element is called its valency.
Please follow below link: Valency
Answered by | 01 Dec, 2023, 09:55: AM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by karmvirsingh9602741719 | 17 May, 2024, 11:42: AM
CBSE 10 - Chemistry
Asked by yadavparmit83 | 01 Dec, 2023, 06:16: AM
CBSE 10 - Chemistry
Asked by ramjilal01071988 | 14 Oct, 2023, 20:42: PM
CBSE 10 - Chemistry
Asked by dikshantnaik1008 | 21 Jul, 2023, 11:47: AM
CBSE 10 - Chemistry
Asked by kashviS.shah | 14 Sep, 2022, 23:27: PM
CBSE 10 - Chemistry
Asked by saharupa28041 | 30 Jun, 2022, 23:29: PM
CBSE 10 - Chemistry
Asked by Sunita | 23 Feb, 2022, 18:25: PM
CBSE 10 - Chemistry
Asked by labheshvaidya | 11 Feb, 2022, 17:01: PM
CBSE 10 - Chemistry
Asked by sssaibadreeshwar | 27 Nov, 2021, 16:07: PM
CBSE 10 - Chemistry
Asked by tdeeksha3109 | 09 Nov, 2021, 14:11: PM