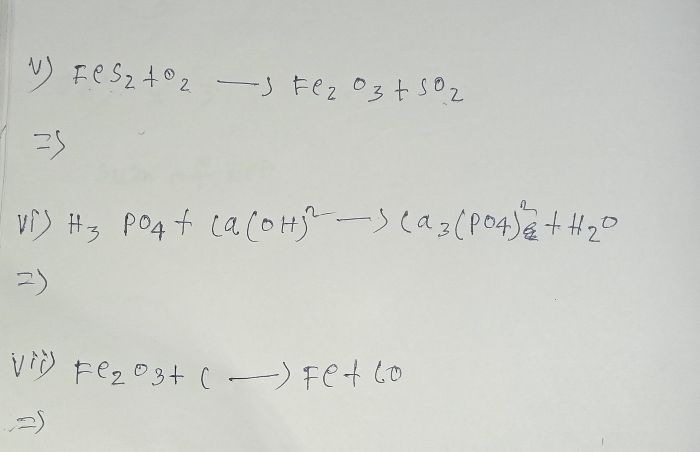CBSE Class 10 Answered
write the balanced chemical equations for the following reaction and identify the type of reaction in each case:
1.nitrogen gas is treated with hydrogen gas in the presence of a catalyst at 773k to from ammonia gas.
2.sodium hydroxide solution is teated with acetic acid to from sodium acetate and water.
3.ethanol is warmed with ethanoic acid to form ethyl acetate in presence of concentration h2so4.
4.ethene is burnt in presence of oxygen to form carbon dioxide,water and release heat and light.
Asked by manya.bhardwaj.01 | 30 Jun, 2017, 04:41: PM
Dear manya.bhardwaj.01@gmail.com
Thanks for asking us a question in Ask the Expert section of TopperLearning.com.
We cannot entertain more than 4 questions in a single query per day. In case of multiple questions within a query, please post each question individually and let us know where you are getting stuck so that we would be able to explain things better.
Please refer the solution to your first question given below:
It is a combination reaction.
Regards
Topperlearning Team.
Topperlearning Team.
Answered by Vaibhav Chavan | 30 Jun, 2017, 06:41: PM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by yadavparmit83 | 01 Dec, 2023, 06:16: AM
CBSE 10 - Chemistry
Asked by ramjilal01071988 | 14 Oct, 2023, 08:42: PM
CBSE 10 - Chemistry
Asked by dikshantnaik1008 | 21 Jul, 2023, 11:47: AM
CBSE 10 - Chemistry
Asked by kashviS.shah | 14 Sep, 2022, 11:27: PM
CBSE 10 - Chemistry
Asked by saharupa28041 | 30 Jun, 2022, 11:29: PM
CBSE 10 - Chemistry
Asked by Sunita | 23 Feb, 2022, 06:25: PM
CBSE 10 - Chemistry
Asked by labheshvaidya | 11 Feb, 2022, 05:01: PM
CBSE 10 - Chemistry
Asked by sssaibadreeshwar | 27 Nov, 2021, 04:07: PM
CBSE 10 - Chemistry
Asked by tdeeksha3109 | 09 Nov, 2021, 02:11: PM
CBSE 10 - Chemistry
Asked by simratkaurr1 | 07 Jun, 2021, 12:55: PM