CBSE Class 11-science Answered
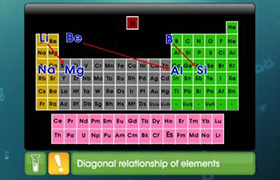
What is the relationship between diagonal relationship of an element and the polarizing power of an element?
Asked by Topperlearning User | 14 Aug, 2014, 13:15: PM
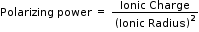
On moving along a period, the ionic charge increases while ionic size decreases so polarizing power increases. On moving down the group the ionic size increases and hence polarizing power decreases. On moving diagonally these two effects cancel each other to some extent and hence the properties remain similar.
Answered by | 14 Aug, 2014, 15:15: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by negimanish9735 | 28 Oct, 2020, 09:30: AM
CBSE 11-science - Chemistry
Asked by abhishhadnur | 28 Sep, 2020, 16:04: PM
CBSE 11-science - Chemistry
Asked by ingale.vishakha08 | 26 Jan, 2019, 14:27: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 14 Aug, 2014, 13:24: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 19 Apr, 2016, 12:30: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 27 Jun, 2016, 13:02: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 14 Aug, 2014, 13:15: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 10 Sep, 2014, 15:33: PM