CBSE Class 11-science Answered
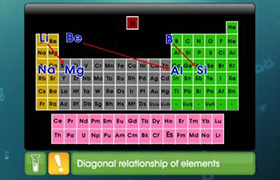
Explain with the help of chemical reaction that MgO is basic oxide while P4O10 is an acidic oxide
Asked by Topperlearning User | 27 Jun, 2016, 13:02: PM
MgO reacts with aqueous HCl to form salt and water thus exhibiting its basic character
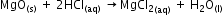
P4O10 reacts with water to form phosphoric acid
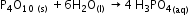
The solution turns blue litmus red.
Answered by | 27 Jun, 2016, 15:02: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by negimanish9735 | 28 Oct, 2020, 09:30: AM
CBSE 11-science - Chemistry
Asked by abhishhadnur | 28 Sep, 2020, 16:04: PM
CBSE 11-science - Chemistry
Asked by ingale.vishakha08 | 26 Jan, 2019, 14:27: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 14 Aug, 2014, 13:24: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 19 Apr, 2016, 12:30: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 27 Jun, 2016, 13:02: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 14 Aug, 2014, 13:15: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 10 Sep, 2014, 15:33: PM