ICSE Class 10 Answered
What is ionisation? Please explain in easy language. Why does ionisation take place? How can covalent compounds be converted into ions as they are composed of molecules?
Asked by 21janhvi.verma | 06 Jun, 2018, 10:29: AM
Ionisation: It is the process of formation of ions from ionic compounds.
For example NaCl
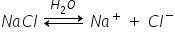
As you can see this process is reversible because they are bonded by the electrostatic force of attraction. hence they form ions on ionisation.
Let's move on to next question of dissociation of covalent compounds.
Let's take an example of HCl,
HCl is formed by sharing the pair of electrons, but due to more electronegativity difference between H and Cl the bond becomes polar covalent bonds and the compound becomes the polar covalent compound.
Let's see its dissociation,
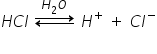
This happens because the OH- ion from water molecule will attract the Cl- ion of HCl hence as both are oppositely charged they attract towards each other and bond between H-Cl is broken and dissociates to form ions.
Answered by Ramandeep | 06 Jun, 2018, 12:26: PM
Application Videos
Concept Videos
ICSE 10 - Chemistry
Asked by navedsheikh97658 | 01 Nov, 2023, 16:57: PM
ICSE 10 - Chemistry
Asked by adityagogineni15.10spicertl | 18 Jun, 2020, 14:01: PM
ICSE 10 - Chemistry
Asked by dhruvasavaliya7916.10sdatl | 24 Apr, 2020, 21:48: PM
ICSE 10 - Chemistry
Asked by rohitmodi785 | 10 Dec, 2019, 09:28: AM
ICSE 10 - Chemistry
Asked by Babyka111 | 03 Jun, 2019, 15:24: PM
ICSE 10 - Chemistry
Asked by mittaljeevesh | 11 Dec, 2018, 19:06: PM
ICSE 10 - Chemistry
Asked by alshivakumar12 | 03 Jul, 2018, 18:11: PM
ICSE 10 - Chemistry
Asked by Nikhil | 12 Jun, 2018, 07:21: AM
ICSE 10 - Chemistry
Asked by 21janhvi.verma | 06 Jun, 2018, 10:29: AM
ICSE 10 - Chemistry
Asked by Shreya | 13 Mar, 2018, 10:05: AM