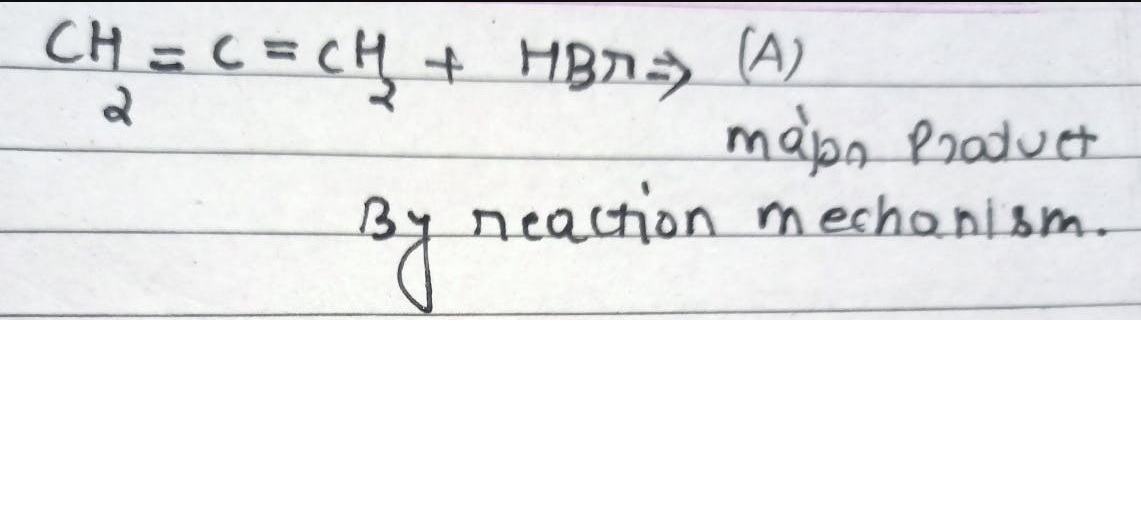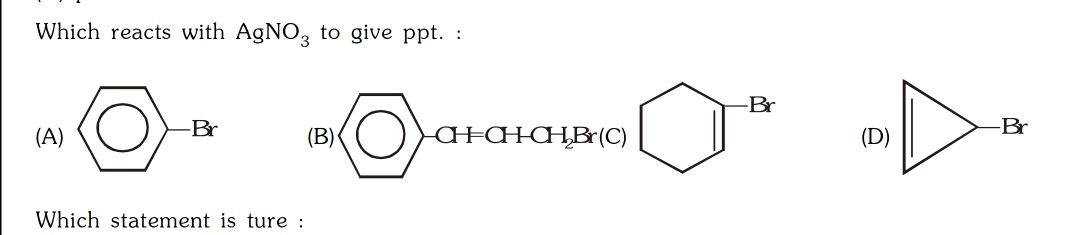CBSE Class 12-science Answered
What is aryl halide? And what is difference between Sn1 and Sn2 reaction?
Asked by akpanda729125 | 19 Jul, 2016, 10:28: PM
In organic chemistry, an aryl halide (also known as haloarene) is an aromatic compound in which one or more hydrogen atoms directly bonded to an aromatic ring are replaced by a halide such as fluoride, chloride, bromide, iodide.
SN1 and SN2 are both nucleophilic substitution reactions, there are some differences:
1. For SN1 reactions, the step determining the rate is unimolecular, whereas for a SN2 reaction, it is bimolecular.
2. SN1 is a two-step mechanism, whereas SN2 is only a one-step process.
3. During SN1 reactions, the carbocation will form as an intermediate, whereas, during SN2 reactions, it is not formed.
4. In SN2 reactions, one can draw the intermediate structure of where the carbon has a partial bond with the incoming nucleophile and the leaving group, whereas this is not possible in SN1 pathway reactions. SN1 reaction: SN2 reaction:
Answered by Prachi Sawant | 20 Jul, 2016, 10:48: AM
Application Videos
Concept Videos
CBSE 12-science - Chemistry
Asked by surajbhanupatro44 | 07 Nov, 2023, 12:01: AM
CBSE 12-science - Chemistry
Asked by mayamishra9540500880 | 04 Jul, 2022, 07:11: PM
CBSE 12-science - Chemistry
Asked by harshaldpathak | 11 Jun, 2022, 05:37: PM
CBSE 12-science - Chemistry
Asked by amitkumar.cis | 01 Jan, 2021, 09:15: PM
CBSE 12-science - Chemistry
Asked by me.mirzainayat | 14 Nov, 2020, 07:31: AM
CBSE 12-science - Chemistry
Asked by Prachidewangan74 | 02 Oct, 2020, 03:02: PM
CBSE 12-science - Chemistry
Asked by sujithanathan119 | 01 Jun, 2020, 12:00: PM
CBSE 12-science - Chemistry
Asked by ng9045007209 | 21 May, 2020, 07:47: PM
CBSE 12-science - Chemistry
Asked by gangavaramouni | 26 Mar, 2020, 10:33: AM