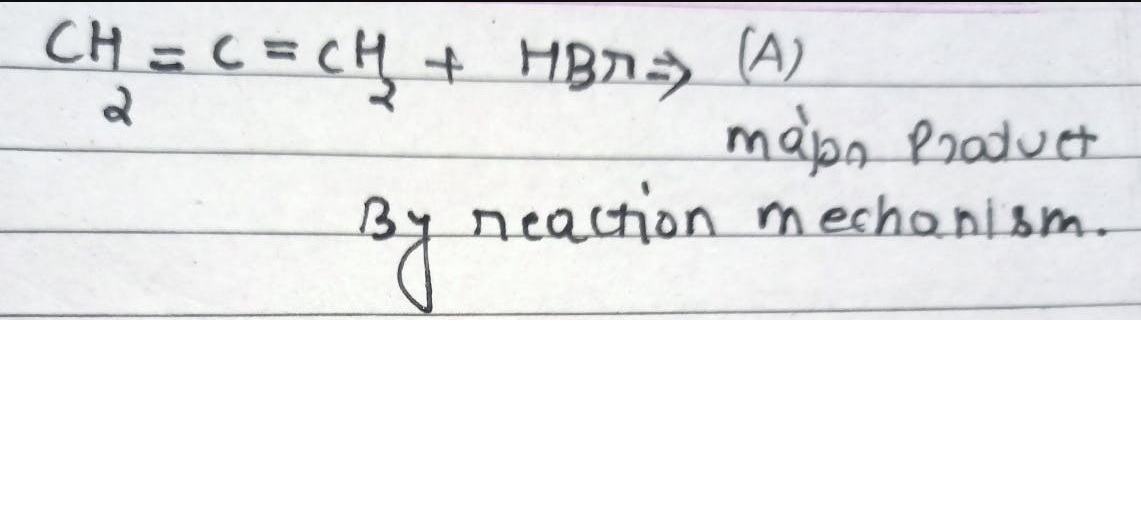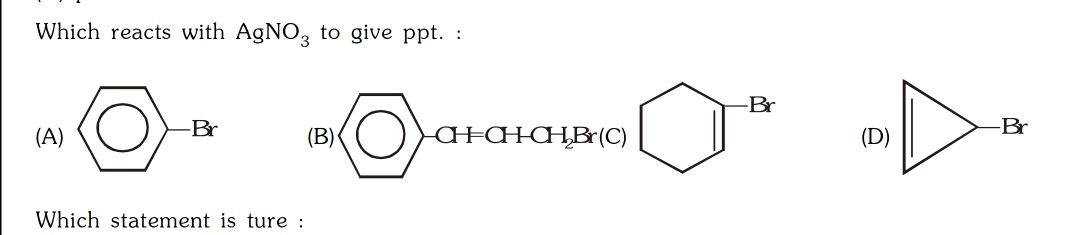CBSE Class 12-science Answered
find the major product

Asked by mayamishra9540500880 | 04 Jul, 2022, 19:11: PM
The given alkene is 3-bromo-2-pentene.
Both carbons in this ethene are attached to one alkyl group which an electron donating group.
But the first carbon is attached to bromide ion which is an electron withdrawing ion.
It results into partial positive charge on the carbon 3 which also attached to ethyl group.
Also, tertiary carbon radical formed at carbon 3 is more stable than secondary carbon radical which can form at carbon 2.
And secondary carbanion at carbon 2 is more stable than tertiary carbanion which can form at carbon 3.
Due to this -I effect by bromide ion, the incoming amine group will get attached to carbon 1 forming, 3-amino-3-bromopentane as major product.
Answered by | 06 Jul, 2022, 19:55: PM
Application Videos
Concept Videos
CBSE 12-science - Chemistry
Asked by surajbhanupatro44 | 07 Nov, 2023, 00:01: AM
CBSE 12-science - Chemistry
Asked by mayamishra9540500880 | 04 Jul, 2022, 19:11: PM
CBSE 12-science - Chemistry
Asked by harshaldpathak | 11 Jun, 2022, 17:37: PM
CBSE 12-science - Chemistry
Asked by amitkumar.cis | 01 Jan, 2021, 21:15: PM
CBSE 12-science - Chemistry
Asked by me.mirzainayat | 14 Nov, 2020, 07:31: AM
CBSE 12-science - Chemistry
Asked by Prachidewangan74 | 02 Oct, 2020, 15:02: PM
CBSE 12-science - Chemistry
Asked by sujithanathan119 | 01 Jun, 2020, 12:00: PM
CBSE 12-science - Chemistry
Asked by ng9045007209 | 21 May, 2020, 19:47: PM
CBSE 12-science - Chemistry
Asked by gangavaramouni | 26 Mar, 2020, 10:33: AM