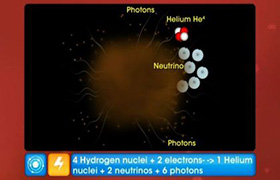
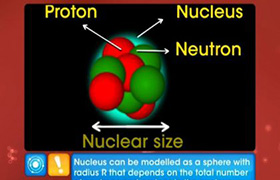
CBSE Class 12-science Answered
WHAT ARE ISOTONES AND ISOTOPES CORRECT DEFINITION?
Asked by sharonashoksp | 27 Jun, 2021, 14:44: PM
Isotones :-
Elements having same number of neutrons but differ in number of protons are called Isotones.
example :- 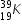 and
and 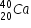
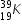 and
and 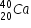
In above example, atmic number of Pottasium is 19 and mass number is 39 . Hence 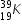 has 19 protons and 20 neutrons
has 19 protons and 20 neutrons
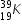 has 19 protons and 20 neutrons
has 19 protons and 20 neutronsAtomic number of Ca is 20 and its mass number is 40. Hence 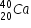 has 20 protons and 20 neutrons.
has 20 protons and 20 neutrons.
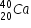 has 20 protons and 20 neutrons.
has 20 protons and 20 neutrons.Since both these elements have same number of neutrons, these elements are called isotones .
---------------------------------------------------------------------
Isotopes :-
Elements having same number of protons but differ in number of neutrons are called Isotopes.
example :- 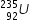 and
and 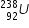
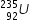 and
and 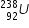
In above example , atomic number of Uranium is 92 , hence both of these elements have same 92 protons ,
but their neutron numbers differ.
Answered by Thiyagarajan K | 27 Jun, 2021, 15:59: PM
Concept Videos
CBSE 12-science - Physics
Asked by mohapatraswetalina88 | 21 Apr, 2024, 12:18: PM
CBSE 12-science - Physics
Asked by rohandhawaniya17112006 | 06 Mar, 2024, 15:32: PM
CBSE 12-science - Physics
Asked by akashjyani705 | 06 Mar, 2022, 16:39: PM
CBSE 12-science - Physics
Asked by sharonashoksp | 27 Jun, 2021, 14:44: PM
CBSE 12-science - Physics
Asked by gaurish6247 | 07 Apr, 2021, 17:16: PM
CBSE 12-science - Physics
Asked by prerna.naga | 09 May, 2019, 08:51: AM
CBSE 12-science - Physics
Asked by kumarisakshi0209 | 17 Mar, 2019, 14:54: PM
CBSE 12-science - Physics
Asked by Amandeepsinghbedi26 | 26 Sep, 2018, 13:52: PM
CBSE 12-science - Physics
Asked by Topperlearning User | 28 May, 2015, 09:51: AM
CBSE 12-science - Physics
Asked by Topperlearning User | 28 May, 2015, 09:53: AM