CBSE Class 12-science Answered
Nuclear density of hydrogen is 2.3x1017 kg/m3. Given A = 56 for iron, find its nuclear density
Asked by gaurish6247 | 07 Apr, 2021, 17:16: PM
Radius of Hydrogen nucleus, RH = Ro A1/3 = Ro
Where A is Mass number
Radius of Fe nucleus RFe = Ro 561/3
Density of hydrogen nuclei , 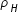 = mass / volume = mH / [ (4/3)π RH3 ]
= mass / volume = mH / [ (4/3)π RH3 ]
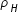 = mass / volume = mH / [ (4/3)π RH3 ]
= mass / volume = mH / [ (4/3)π RH3 ]Density of Fe nuclei , 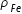 = mFe / [ (4/3)π RFe3 ]
= mFe / [ (4/3)π RFe3 ]
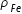 = mFe / [ (4/3)π RFe3 ]
= mFe / [ (4/3)π RFe3 ]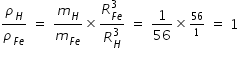
Density of Fe nucleus and Density of Hydrogen nucleus are same
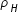 =
= 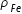 = 2.3 × 1017 kg/m3
= 2.3 × 1017 kg/m3
Answered by Thiyagarajan K | 07 Apr, 2021, 22:11: PM
Concept Videos
CBSE 12-science - Physics
Asked by mohapatraswetalina88 | 21 Apr, 2024, 12:18: PM
CBSE 12-science - Physics
Asked by rohandhawaniya17112006 | 06 Mar, 2024, 15:32: PM
CBSE 12-science - Physics
Asked by akashjyani705 | 06 Mar, 2022, 16:39: PM
CBSE 12-science - Physics
Asked by sharonashoksp | 27 Jun, 2021, 14:44: PM
CBSE 12-science - Physics
Asked by gaurish6247 | 07 Apr, 2021, 17:16: PM
CBSE 12-science - Physics
Asked by prerna.naga | 09 May, 2019, 08:51: AM
CBSE 12-science - Physics
Asked by kumarisakshi0209 | 17 Mar, 2019, 14:54: PM
CBSE 12-science - Physics
Asked by Amandeepsinghbedi26 | 26 Sep, 2018, 13:52: PM
CBSE 12-science - Physics
Asked by Topperlearning User | 28 May, 2015, 09:51: AM
CBSE 12-science - Physics
Asked by Topperlearning User | 28 May, 2015, 09:53: AM