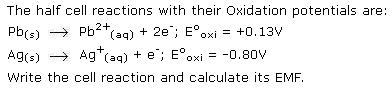CBSE Class 11-science Answered
The standard electrode potentials of a few metals are given below:
AI(-0.66V), Cu(+0.34V), Li(-3.05V), Ag(+0.80V) and Zn(-0.76V)
Which of these will behave as the strongest oxidizing agent and which as the strongest reducing agent?
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
Li is the strongest reducing agent while Ag+ is the strongest oxidizing agent.
Answered by | 04 Jun, 2014, 15:23: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by govtsecschoolnayaganv051 | 16 Sep, 2018, 21:26: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 14 Jun, 2016, 11:01: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM