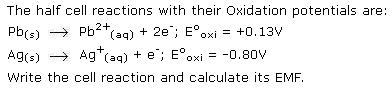CBSE Class 11-science Answered
I2 and Br2 are added to a solution containing Br- and I- ions. What reaction will occur if, I2 + 2e- 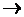 2I-; E0 = +0.54 V and Br2 + 2e-
2I-; E0 = +0.54 V and Br2 + 2e- 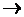 2Br-; E0 = +1.09 V?
2Br-; E0 = +1.09 V?
Asked by Topperlearning User | 14 Jun, 2016, 11:01: AM
Since E0 of Br2 is higher than that of I2, therefore, Br2 has a higher tendency to accept electrons that I2. Conversely, I- ion has a higher tendency to lose electrons than Br- ions. Therefore, the following reaction will occur:
2I- 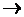 I2 + 2e-
I2 + 2e-
Br2 +2e- 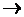 2Br-
2Br-
2I- + Br2 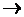 I2 +2Br-
I2 +2Br-
In other words I- ion will be oxidized to I2 while Br2 will be reduced to Br- ion.
Answered by | 14 Jun, 2016, 13:01: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by govtsecschoolnayaganv051 | 16 Sep, 2018, 21:26: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 14 Jun, 2016, 11:01: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM