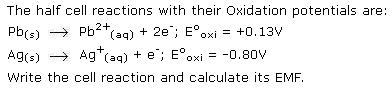CBSE Class 11-science Answered
Ques1) Standard electrode potentials are
Fe 2+ / Fe ( E° = -0.44 V)
Fe 3+ / Fe2+ (E° = 0.77 V)
If Fe 2+, Fe 3+ and Fe blocks are kept together , then
(a) Fe3+ increases (b) Fe3+ decreases
(c) Fe2+ / Fe3+ remains unchanged (d) Fe2+ decreases
please give explaination as I know the answer is (b) but don't know the reason
Asked by govtsecschoolnayaganv051 | 16 Sep, 2018, 21:26: PM
The cell formed will be :
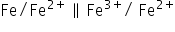
Eºcell = 0.44 + 0.77 = 1.21 V
Metals having higher negative values of their electrode potential can displace metal having lower values from their salt solution
Therefore the magnitude of Fe3+ ions will decrease.
Answered by Ramandeep | 17 Sep, 2018, 12:07: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by govtsecschoolnayaganv051 | 16 Sep, 2018, 21:26: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 14 Jun, 2016, 11:01: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM