CBSE Class 11-science Answered
the ratio of potential energy of electron in the 4th orbit of 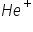 ion to the kinetic energy of electron in 3rd orbit of
ion to the kinetic energy of electron in 3rd orbit of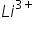 ion would be????
ion would be????
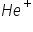 ion to the kinetic energy of electron in 3rd orbit of
ion to the kinetic energy of electron in 3rd orbit of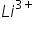 ion would be????
ion would be????
Asked by Anu | 19 Jun, 2014, 12:28: PM
The equation of P.E for a one electron atom is
Ep = - Z2e4m
4e02 h2n2
So e- in He+ ion in 4th orbit
Ep = - 22e4m = -me4
4e02 h242 16e02 h2
The equation of K.E for a one electron atom is
Ek = Z2e4m
8e02 h2n2
So e- in Li+3 ion in 3rd orbit
Ek = 32e4m = me4
8e02 h232 8e02 h2
Ep = -me4 x 8 e02 h2 = -1
Ek 16e02 h2 x me4 2
Answered by Vaibhav Chavan | 20 Jun, 2014, 05:35: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by o230397 | 23 Sep, 2023, 02:48: PM
CBSE 11-science - Chemistry
Asked by ks1221516 | 14 Nov, 2021, 06:46: PM
CBSE 11-science - Chemistry
Does shielding effect occur between atomic orbitals of the same quantum number? Explain with reasons
Asked by fishtailfever | 08 Sep, 2019, 11:00: PM
CBSE 11-science - Chemistry
Asked by patra04011965 | 09 Aug, 2019, 05:19: PM
CBSE 11-science - Chemistry
Asked by bhrjitu | 21 Jul, 2019, 08:08: AM
CBSE 11-science - Chemistry
Asked by pb_ckt | 06 Jun, 2019, 09:15: AM
CBSE 11-science - Chemistry
Asked by shahrithik07 | 18 Oct, 2018, 05:26: PM
CBSE 11-science - Chemistry
Asked by chinjalsoni911 | 16 Oct, 2018, 06:14: PM
CBSE 11-science - Chemistry
Asked by arunavamitra50 | 18 Jun, 2018, 06:39: PM
CBSE 11-science - Chemistry
Asked by badalsharma9929 | 13 Jun, 2018, 08:43: AM