CBSE Class 11-science Answered
The hydrated salt na2so4 nh20 under goes 55% loss in weight on heating and become anhydrous.the value of small n? will
Asked by sireeshabandaru88 | 11 Apr, 2020, 12:24: PM
Molar mass of Na2SO4 = 142 gm
Percent loss of water from Na2SO4 is 55
So,
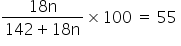
1800n = 7810 + 990n
n =9.64
n≈ 10 mol
Answered by Varsha | 11 Apr, 2020, 17:34: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by shreekrishnampatil | 27 Apr, 2024, 22:31: PM
CBSE 11-science - Chemistry
Asked by jayag1298 | 08 Apr, 2024, 15:14: PM
CBSE 11-science - Chemistry
Asked by omniscientnjf2021 | 07 Apr, 2024, 22:18: PM
CBSE 11-science - Chemistry
Asked by hcnainwal | 15 Jun, 2023, 10:39: AM
CBSE 11-science - Chemistry
Asked by Jprmumal29 | 18 Dec, 2022, 21:48: PM
CBSE 11-science - Chemistry
Asked by mallikarjunasangi28 | 22 Jul, 2022, 19:57: PM
CBSE 11-science - Chemistry
Asked by vedwatisharma79 | 10 Jun, 2022, 17:27: PM
CBSE 11-science - Chemistry
Asked by thathvakunjusree | 10 Dec, 2021, 06:46: AM
CBSE 11-science - Chemistry
Asked by udheshraddha2004 | 28 Oct, 2021, 21:37: PM
CBSE 11-science - Chemistry
Asked by arunparewa2000 | 27 Oct, 2021, 18:59: PM







