CBSE Class 11-science Answered
324g of 20% by mass of Ba(OH)2 solution

Asked by arunparewa2000 | 27 Oct, 2021, 18:59: PM
The mass of Ba(OH)2 is 342 g.
The molar mass of Ba(OH)2 is 171 gm
Hence, 1 mole of Ba(OH)2 is the molar mass of Ba(OH)2 is 171 gm
So, for 342 g. wil be x
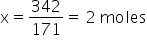
We know 100% by mass is 2moles hence 20% will be x.
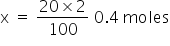
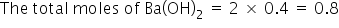
As given the molarity of HNO3 is 2M, So, the total moles=
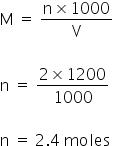
Total acidic excess moles = 2.4 - 0.8 = 1.6
The total volume of Ba(OH)2 is 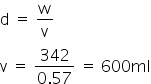
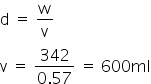
The volume of HNO3 is 1200ml
Hence the total volume of Ba(OH)2 = 1200+600 =1800 ml
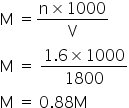
Answered by Ramandeep | 28 Oct, 2021, 14:50: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by shreekrishnampatil | 27 Apr, 2024, 22:31: PM
CBSE 11-science - Chemistry
Asked by jayag1298 | 08 Apr, 2024, 15:14: PM
CBSE 11-science - Chemistry
Asked by omniscientnjf2021 | 07 Apr, 2024, 22:18: PM
CBSE 11-science - Chemistry
Asked by hcnainwal | 15 Jun, 2023, 10:39: AM
CBSE 11-science - Chemistry
Asked by Jprmumal29 | 18 Dec, 2022, 21:48: PM
CBSE 11-science - Chemistry
Asked by mallikarjunasangi28 | 22 Jul, 2022, 19:57: PM
CBSE 11-science - Chemistry
Asked by vedwatisharma79 | 10 Jun, 2022, 17:27: PM
CBSE 11-science - Chemistry
Asked by thathvakunjusree | 10 Dec, 2021, 06:46: AM
CBSE 11-science - Chemistry
Asked by udheshraddha2004 | 28 Oct, 2021, 21:37: PM
CBSE 11-science - Chemistry
Asked by arunparewa2000 | 27 Oct, 2021, 18:59: PM







